Heterocyclic compounds form a cornerstone of organic chemistry, influencing numerous fields from pharmaceuticals to materials science. Among these, pyrrole, furan, and thiophene stand out due to their unique structures and significant chemical properties. These compounds, characterized by five-membered rings, differ subtly in composition but significantly impact their applications and behaviors.
Pyrrole, furan, and thiophene are aromatic heterocycles, each containing a five-membered ring but differing in the heteroatom present: nitrogen in pyrrole, oxygen in furan, and sulfur in thiophene. These differences lead to distinct chemical reactivities and physical properties which are pivotal in their various industrial uses. Understanding these distinctions is key to leveraging their potential in scientific and industrial applications.
Their presence is pervasive in many synthetic and natural products, where they are valued for their electronic properties and contributions to stability and reactivity. Exploring these compounds illuminates not only the fundamental principles of aromaticity but also the intricacies of molecular interaction that define much of modern chemistry.
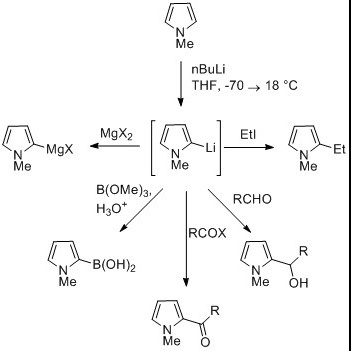
Pyrrole Overview
Basic Structure
Pyrrole is a fascinating molecule in the realm of organic chemistry, particularly among heterocyclic compounds. At its core, pyrrole consists of a five-membered ring containing four carbon atoms and one nitrogen atom. The presence of nitrogen introduces a lone pair of electrons, contributing to the aromatic nature of pyrrole. This structure allows it to engage in pi-stacking and hydrogen bonding, crucial for its stability and reactivity.
Chemical Properties
Pyrrole is notably electron-rich due to the nitrogen’s lone pair of electrons, which are part of the aromatic sextet. This characteristic makes pyrrole an electron donor in various chemical reactions, particularly in the formation of coordination complexes with metals. Its reactivity includes:
- Nucleophilic Substitution: Pyrrole reacts at the 2-position due to the electron-donating effect of the nitrogen.
- Electrophilic Substitution: Typically occurs at the 2 and 5 positions, influenced by the aromatic system and the nitrogen’s electron donation.
Furan Overview
Basic Structure
Furan features a five-membered ring like pyrrole but with an oxygen atom replacing the nitrogen. This oxygen atom significantly influences the electronic characteristics of furan, making it less electron-rich due to oxygen’s higher electronegativity compared to nitrogen. The structure of furan includes four carbon atoms and one oxygen atom, contributing to its unique properties among oxygen-containing heterocycles.
Chemical Properties
Furan is characterized by its reactivity towards electrophilic agents, a trait driven by the aromatic pi-electron cloud that is slightly more available due to the oxygen atom. Key reactions include:
- Oxidation: Furan rings can be easily oxidized, leading to the formation of diketones or ring-opening products.
- Electrophilic Substitution: Generally occurs at the alpha-position relative to oxygen due to the influence of the aromatic system.

Thiophene Overview
Basic Structure
Thiophene, another member of the five-membered heterocyclic family, includes a sulfur atom instead of nitrogen or oxygen. This sulfur atom imparts a unique electronic environment, making thiophene relatively more stable than its counterparts. The ring is composed of four carbon atoms and one sulfur atom, which shares properties with both benzene and pyrrole due to its hybrid aromatic character.
Chemical Properties
The presence of sulfur in thiophene enhances its electron density, making it resistant to electrophilic substitution compared to furan. Its chemical behavior is distinguished by:
- Electrophilic Substitution: Occurs less readily than in furan or pyrrole, typically at positions 2 and 5.
- Nucleophilic Reactions: These are less common but can occur under specific conditions, driven by the sulfur atom’s influence.
Structural Differences
Ring Structure Comparison
While pyrrole, furan, and thiophene all share a five-membered ring structure, the heteroatom (nitrogen in pyrrole, oxygen in furan, and sulfur in thiophene) plays a pivotal role in defining their structural and electronic differences. This difference significantly impacts their physical properties and reactivity, a key consideration in their respective applications.
Atoms and Bonding
The type of heteroatom affects the electron distribution across the ring. Nitrogen in pyrrole donates electron density due to its lone pair, oxygen in furan withdraws electron density due to its electronegativity, and sulfur in thiophene provides a balance between electron donation and withdrawal, stabilizing the ring.
Chemical Properties
Reactivity Patterns
Each of these compounds exhibits unique reactivity patterns influenced by the nature of the heteroatom:
- Pyrrole: Prefers reactions that stabilize the lone pair on nitrogen.
- Furan: Shows significant reactivity towards oxidation due to the oxygen atom.
- Thiophene: Exhibits stability that makes it less reactive towards electrophilic substitution.
Stability and Aromaticity
The aromaticity of these compounds is crucial for their stability. Pyrrole’s aromaticity is enhanced by nitrogen’s lone pair, furan’s aromaticity is moderated by oxygen, and thiophene’s stability is boosted by sulfur’s ability to contribute to the pi-electron system. This feature is essential in their use in polymers, drugs, and electronic materials, where stability under various conditions is paramount.
Physical Properties
Boiling Points and Melting Points
The physical properties of pyrrole, furan, and thiophene, such as boiling points and melting points, vary due to their different heteroatoms and resulting electronic structures. Pyrrole has a boiling point of about 130°C and a melting point of -23°C, making it a liquid at room temperature. Furan is more volatile with a boiling point of 32°C and a melting point of -85.6°C. Thiophene exhibits a higher boiling point of 84°C and a melting point of -38°C. These properties affect how each compound is handled and used in industrial applications, emphasizing the need for careful temperature control during their synthesis and use.
Solubility and Density
Solubility and density also highlight differences among these compounds:
- Pyrrole is moderately soluble in water and more so in organic solvents, facilitating its use in various chemical reactions and industrial applications.
- Furan is slightly soluble in water but highly soluble in organic solvents, which is advantageous for pharmaceutical syntheses.
- Thiophene, similar to furan, has limited water solubility but excellent solubility in most organic solvents, useful in polymer and material science applications.
The density of these compounds generally reflects their molecular weight and structural compactness, influencing their behavior in mixtures and reactions.
Applications in Industry
Use in Pharmaceuticals
Pyrrole, furan, and thiophene derivatives play crucial roles in the pharmaceutical industry. They are foundational in creating compounds with biological activity, such as:
- Pyrrole: Used in the synthesis of drugs that treat arrhythmias and inflammation.
- Furan: Found in antibacterial and antifungal medications.
- Thiophene: Incorporated into drugs that manage diabetes and central nervous system disorders.
Their ability to interact with various biological targets makes these heterocycles valuable in drug design and synthesis.
Role in Material Science
In material science, the properties of these heterocycles facilitate the development of high-performance materials:
- Pyrrole: Integral to conducting polymers used in electronic devices.
- Furan: Employed in resins and composites that require high thermal stability.
- Thiophene: Essential for organic semiconductors and photovoltaic cells.
Their structural versatility allows engineers to tailor materials for specific electronic or mechanical properties.
Synthesis Methods
Pyrrole Synthesis
The synthesis of pyrrole can be achieved through several methods, but the most common is the Knorr synthesis, which involves reacting ketones with ammonia and alpha-amino acids. This method produces pyrrole through condensation and cyclization reactions, useful in large-scale productions.
Furan Synthesis
Furan is typically synthesized from carbohydrates via the Furfural route, where pentose sugars are dehydrated in the presence of acidic catalysts. This method highlights the conversion of renewable resources into valuable chemical products.
Thiophene Synthesis
Thiophene synthesis often employs the Gattermann-Thiophene synthesis, which involves the cyclization of 1,4-diketones under acidic conditions. This process illustrates the transformation of simple organic molecules into more complex and functional heterocyclic structures.
Environmental Impact
Degradation and Toxicity
Environmental considerations are crucial when dealing with pyrrole, furan, and thiophene:
- Pyrrole degrades under exposure to light and air, reducing its environmental persistence but raising concerns about its metabolites.
- Furan is more concerning due to its potential toxicity and carcinogenicity, particularly when it forms part of the human diet as a food contaminant.
- Thiophene derivatives vary in their stability and degradation pathways, impacting environmental safety and degradation products.
Regulations and Safety
Regulations governing the use and disposal of these heterocycles are stringent, reflecting their potential health and environmental risks. Handling practices must adhere to safety protocols to minimize exposure and prevent contamination. Safety data sheets and regulatory guidelines are critical resources for industries that manufacture or use these chemicals, ensuring that all procedures align with environmental and health standards.
Frequently Asked Questions
What is Aromaticity?
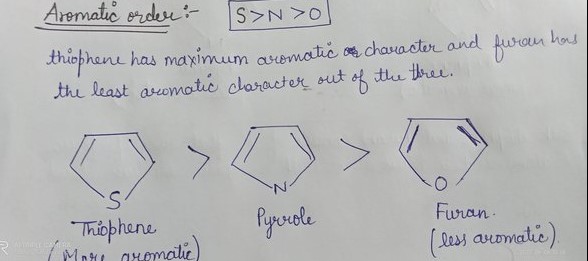
Aromaticity refers to the stability provided by the delocalized pi electrons in certain cyclic compounds. Pyrrole, furan, and thiophene are considered aromatic because they conform to Huckel’s rule, having (4n+2) pi electrons, which contributes to their chemical stability and reactivity.
How are Pyrrole, Furan, and Thiophene Synthesized?
These heterocycles are typically synthesized through various organic reactions. Pyrrole can be synthesized from amino acids or ketones, furan from pentose sugars through dehydration, and thiophene from 1,4-diketones under acidic conditions. Each synthesis method highlights the adaptability and accessibility of these compounds in laboratory and industrial settings.
What are the Industrial Uses of Thiophene?
Thiophene and its derivatives are extensively used in the synthesis of polymers and organic semiconductors. Its sulfur component offers unique electronic properties, making it ideal for applications in photovoltaic cells and as a building block in conductive materials.
Why are Furan Compounds Important in Pharmaceuticals?
Furan derivatives are crucial in pharmaceutical chemistry due to their biological activity. They serve as key intermediates in the synthesis of various drugs, including antibiotics and antifungal agents. Their oxygen-containing ring influences their interaction with biological systems, enhancing drug efficacy.
Conclusion
Pyrrole, furan, and thiophene embody the elegance and complexity of heterocyclic chemistry. Each of these compounds contributes uniquely to scientific advancements and industrial applications, underlined by their distinct structural and chemical properties. Their ongoing study not only enriches our understanding of chemical interactions but also opens new avenues for innovation across multiple disciplines.
The exploration of these compounds is far from complete. As researchers continue to uncover the nuances of their behavior and potential applications, the breadth of their impact is expected to expand, promising new technologies and enhancements in various fields. The future of heterocyclic chemistry continues to be an exciting and fruitful area of inquiry, driven by the foundational roles of pyrrole, furan, and thiophene.

