Xylene, a significant aromatic hydrocarbon, plays a pivotal role in numerous industrial applications. Comprised of a benzene ring with two methyl substituents, its derivatives are crucial in the manufacturing of plastics, fibers, and other chemicals. The focus here is on two of its isomers: o-xylene and p-xylene, each with unique attributes and uses.
O-xylene and p-xylene are isomers of xylene, distinguished by the positions of methyl groups attached to their benzene rings. O-xylene has its methyl groups at the ortho position, while p-xylene’s methyl groups are at the para position. This structural variance significantly influences their physical properties and applications, making them suitable for different industrial purposes.
Xylene’s isomers are not only foundational in creating materials like polyesters but also serve in producing solvents, dyes, and coatings. Understanding the distinctions between o-xylene and p-xylene is crucial for industries that depend on their specific properties to enhance product performance and efficiency.
Xylene Basics
Chemical Structure and Properties
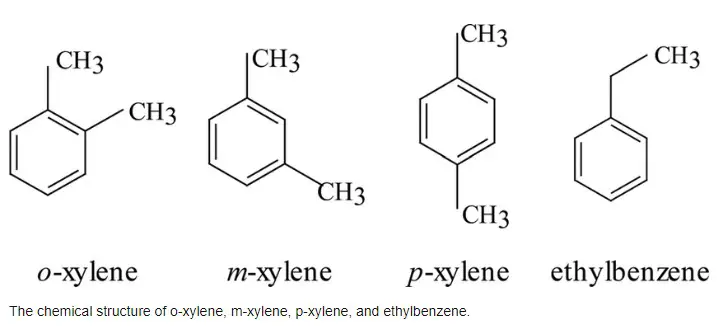
Xylene, a volatile organic compound, is a clear, colorless liquid that is part of the benzene chemical family. The molecular structure of xylene features a benzene ring, which is a ring of six carbon atoms, with two methyl groups attached as substituents. These methyl groups can be positioned differently, leading to three isomeric forms of xylene: ortho-xylene (o-xylene), meta-xylene (m-xylene), and para-xylene (p-xylene).
Each isomer has a molecular formula of C8H10 but differs in the arrangement of the methyl groups on the benzene ring. This variation affects their boiling points, melting points, and solubility, making each isomer suitable for specific applications. Generally, xylenes are known for their high solvency, effective in a range of industrial processes.
Common Uses in Industry
Xylenes are extensively used across various industries due to their solvent properties. They play a critical role in:
- Paints and Coatings: Xylenes are used as solvents in the production of paints and coatings, enhancing the application and drying properties.
- Printing: In printing industries, xylenes are employed to dissolve resins and other components.
- Adhesives: They are used in adhesives for the rubber and leather industries.
- Cleaning Agents: Due to their solvent properties, xylenes are effective in cleaning agents for steel and other metals.
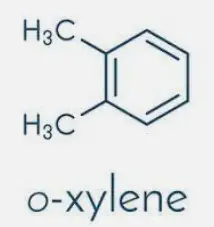
O-Xylene Explained
Chemical Structure
O-xylene, one of the xylene isomers, has its two methyl groups attached to the first and second carbon atoms of the benzene ring, adjacent to each other. This arrangement is termed the ortho configuration.
Physical Properties
O-xylene exhibits specific physical characteristics:
- Boiling Point: Approximately 144°C
- Density: About 0.88 g/cm³ at 20°C
- Solubility: Slightly soluble in water but highly soluble in alcohols and ethers.
Key Industrial Applications
O-xylene is pivotal in the production of:
- Phthalic Anhydride: This chemical is essential for making plasticizers and unsaturated polyester resins.
- Dyes and Pigments: It serves as a precursor in synthesizing various dyes and pigments.
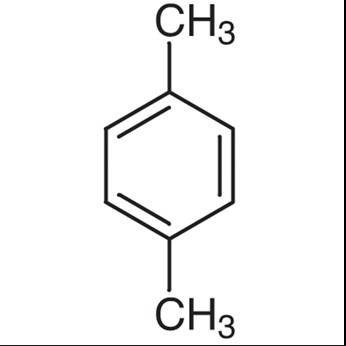
P-Xylene Detailed
Chemical Structure
P-xylene features a chemical arrangement where the methyl groups are located at the first and fourth carbon atoms, known as the para position.
Physical Properties
The physical properties of p-xylene include:
- Boiling Point: Around 138°C
- Density: Approximately 0.86 g/cm³
- Solubility: Like o-xylene, it is slightly soluble in water but dissolves well in organic solvents.
Primary Uses in Manufacturing
P-xylene is primarily used in the manufacture of:
- Terephthalic Acid: A key component in producing polyester fibers and resins.
- Polyethylene Terephthalate (PET): Used extensively in textile and packaging industries.
Comparative Analysis
Structural Differences
The key distinction between o-xylene and p-xylene lies in the placement of the methyl groups on the benzene ring. This difference critically influences their chemical and physical behavior.
Physical and Chemical Property Comparison
- Boiling Points: P-xylene boils at a slightly lower temperature than o-xylene.
- Density and Solubility: Both isomers exhibit similar densities, but their solubility in various solvents can differ due to the arrangement of the methyl groups.
Analysis of Boiling Points, Solubility, and Density
This comparative analysis helps in selecting the appropriate xylene isomer for specific industrial applications, ensuring optimal performance and efficiency in processes.
Production Processes
Synthesis of O-Xylene
O-xylene is typically produced through the distillation of crude xylene mixtures obtained from the refining of petroleum oil. The process involves:
- Fractional Distillation: Separating the xylene isomers based on their boiling points.
- Transalkylation: Adjusting the production ratio of different xylene isomers.
Synthesis of P-Xylene
The production of p-xylene often focuses on maximizing yield due to its demand in the polyester industry. Techniques include:
- Selective Adsorption: Using molecular sieves to isolate p-xylene from mixed xylene streams.
- Catalytic Reforming: Enhancing the output of p-xylene during the petroleum refining process.
Differences in Manufacturing Methods
The production techniques for o-xylene and p-xylene are tailored to meet specific industrial demands and efficiency standards. These methods ensure the availability of each isomer in sufficient quantities and purities for their respective applications.
Application Spectrum
Unique Applications of O-Xylene
O-xylene has specialized uses in various industries due to its unique chemical properties:
- Production of Phthalic Anhydride: This is a primary use where o-xylene is oxidized to produce phthalic anhydride, crucial for making plasticizers used in PVC.
- Synthesis of Herbicides and Insecticides: Its derivatives are employed in the synthesis of agricultural chemicals, enhancing crop protection.
Unique Applications of P-Xylene
P-xylene, distinct from its isomer, finds critical use in:
- Polyester Manufacturing: Serving as a precursor to terephthalic acid and dimethyl terephthalate, p-xylene is vital in the production of polyester fibers used in textiles and PET bottles.
- High-Purity Solvents: Due to its stability, it is also used as a high-purity solvent in research and pharmaceutical industries.
Comparative Analysis in Use Cases
While both isomers serve as precursors in polymer production, their unique chemical structures determine specific end-uses. O-xylene’s role in producing phthalic anhydride contrasts with p-xylene’s crucial involvement in polyester and plastics manufacturing, highlighting their tailored applications in different sectors.
Health and Safety
Exposure Risks for O-Xylene
Handling o-xylene can pose several health risks:
- Inhalation: Breathing in vapors can lead to respiratory issues and dizziness.
- Skin Contact: Direct contact can cause irritation and dermatitis.
Exposure Risks for P-Xylene
P-xylene shares similar risks with its isomer:
- Inhalation Hazards: Long-term exposure can affect the central nervous system.
- Eye and Skin Contact: Can lead to irritation and more severe health problems with prolonged exposure.
Safety Measures and Handling
Implementing strict safety measures is crucial:
- Use of Personal Protective Equipment (PPE): Including gloves, goggles, and respirators.
- Engineering Controls: Such as proper ventilation systems to reduce vapor concentrations in the air.
- Handling Procedures: Training and guidelines to ensure safe storage, handling, and disposal.
Environmental Impact
Environmental Concerns with O-Xylene
O-xylene’s environmental risks involve:
- Water and Soil Contamination: Leaks and spills can contaminate local ecosystems.
- Air Quality: Volatile organic compounds (VOCs) contribute to air pollution and smog.
Environmental Concerns with P-Xylene
Similarly, p-xylene impacts the environment:
- Persistence in the Environment: It can remain in water and soil, affecting aquatic life.
- Contribution to VOCs: Emissions during manufacturing and use add to environmental VOC levels.
Regulatory Overview and Compliance
Regulations such as the Clean Air Act and local environmental guidelines govern the handling and use of xylenes:
- Emission Controls: Requirements to limit VOC emissions from industrial sites.
- Waste Management: Protocols for the disposal and treatment of xylene-containing waste.
Market Dynamics
Global Demand Trends for O-Xylene
The demand for o-xylene is driven by its application in industries such as plastics and chemicals:
- Growth in Plastics Industry: Increasing demand for PVC and related products boosts o-xylene usage.
- Regional Trends: Asia-Pacific leads in consumption due to its expanding industrial base.
Global Demand Trends for P-Xylene
P-xylene’s market dynamics are influenced by the polyester industry:
- Textile Industry Demand: High consumption in textile manufacturing, particularly in China and India.
- Recycling Trends: Growing trends in PET recycling impact fresh p-xylene demand.
Impact on Related Industries
The xylene market affects various sectors:
- Chemical Manufacturing: As a raw material, changes in xylene availability and price affect the chemical industry.
- Automotive and Construction: Industries using paints, adhesives, and coatings also depend on xylene supply stability.
Future Prospects
Research and Development in Xylene Isomers
Innovations focus on more sustainable and efficient production methods:
- Catalytic Processes: Developing more selective catalysts that increase yield and reduce by-products.
- Green Chemistry: Efforts to minimize environmental impact during xylene production.
Emerging Applications and Technologies
New uses and technologies are emerging:
- Advanced Recycling Technologies: Enhancing the recycling of PET to reduce reliance on virgin p-xylene.
- Bio-Based Alternatives: Research into renewable sources for xylene to decrease dependency on petroleum sources.
Predictions for Market Changes
The future of the xylene market looks towards:
- Sustainability Trends: Increasing regulations and market demand for more sustainable practices.
- Market Volatility: Prices and supply chains might see fluctuations due to geopolitical and economic factors, influencing global market trends.
Frequently Asked Questions
What is o-xylene?
O-xylene is an aromatic hydrocarbon known for its two methyl groups attached adjacently on a benzene ring. It is primarily used in the production of phthalic anhydride, which is a precursor to many synthetic dyes, pigments, and resins.
What is p-xylene?
P-xylene is another isomer of xylene with its methyl groups positioned oppositely on the benzene ring. It is extensively used in the manufacture of terephthalic acid and dimethyl terephthalate, key ingredients in the production of polyester.
How are o-xylene and p-xylene produced?
Both o-xylene and p-xylene are derived from the catalytic reforming of petroleum naphtha and the coal carbonization in the manufacture of coke. Specific production methods vary slightly to optimize yield and purity based on the desired isomer.
Are o-xylene and p-xylene dangerous?
Like many industrial chemicals, both o-xylene and p-xylene pose health risks if not handled properly. They can cause respiratory issues, skin irritation, and other health problems. Safety protocols include proper ventilation, use of protective gear, and rigorous handling procedures to mitigate exposure.
How do o-xylene and p-xylene impact the environment?
Environmental impacts of o-xylene and p-xylene include potential risks to water sources and wildlife due to their toxicity and persistence in the environment. Regulations and advanced recovery technologies are crucial to minimize these effects.
Conclusion
In summarizing the characteristics and applications of o-xylene and p-xylene, it becomes evident that these chemicals are integral to various industrial processes. Their unique chemical structures tailor them for specific uses that are critical in the production of numerous consumer goods and industrial materials.
Finally, the ongoing study and understanding of o-xylene and p-xylene not only enhance industrial efficiency but also foster developments in safety and environmental sustainability. As research progresses, it is anticipated that new applications and improved handling practices will emerge, underscoring the importance of these versatile chemical isomers.