Mass measurement plays a pivotal role in various scientific fields, ranging from chemistry and pharmacology to environmental studies. Accurately determining the mass of atoms and molecules is crucial for research and application, particularly when distinguishing between similar substances or understanding their properties. Two specific types of mass measurements, monoisotopic mass and average mass, are fundamental yet distinctly different in their applications and calculation methods.
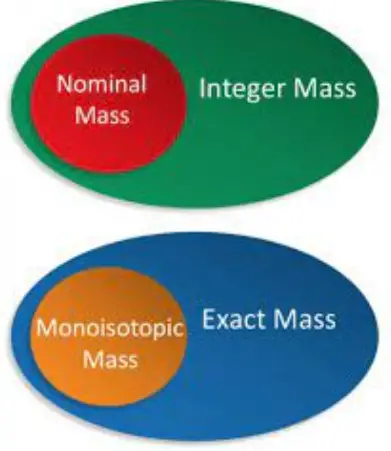
Monoisotopic mass refers to the mass of an atom or molecule that includes the most abundant isotope of each element present, measured in atomic mass units (amu). In contrast, average mass is the weighted average of all isotopic masses of the atoms in a molecule, taking into account their natural abundance. This distinction is vital for precision in scientific calculations and instrumental techniques like mass spectrometry.
Both monoisotopic and average mass are crucial for their respective uses in science. Monoisotopic mass is particularly important for high precision measurements in mass spectrometry, whereas average mass is commonly used in educational contexts and basic chemical calculations. Understanding these differences is essential for professionals and students in scientific fields.
Basic Concepts
Atomic Mass Basics
Definition of Atomic Mass
Atomic mass is a fundamental concept in chemistry, referring to the mass of a single atom, typically expressed in atomic mass units (amu). It is calculated based on a scale where carbon-12 (12C) has an exact mass of 12 amu. Understanding atomic mass is essential for the precise calculation of molecular formulas and reactions.
Importance in Chemistry
The atomic mass is vital for:
- Balancing chemical equations: Knowing the atomic masses of elements allows chemists to balance equations accurately, ensuring the conservation of mass in reactions.
- Molecular weight calculations: The sum of the atomic masses in a molecule gives its molecular weight, crucial for stoichiometry.
- Quantitative analysis: Helps in determining the quantity of a substance involved in reactions.
Isotopes Explained
What are Isotopes?
Isotopes are variants of elements that have the same number of protons but different numbers of neutrons. This difference does not affect the chemical properties significantly but does impact the atomic mass.
Common Isotopes in Elements
Many elements have naturally occurring isotopes. For example:
- Carbon: Carbon-12 and Carbon-13 are stable, while Carbon-14 is radioactive and used in dating organic materials.
- Hydrogen: Protium, Deuterium, and Tritium, where Tritium is radioactive.
Monoisotopic Mass
Defining Monoisotopic Mass
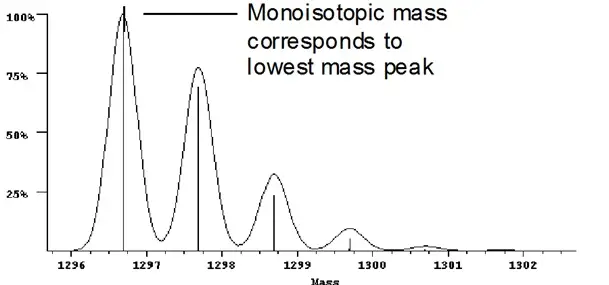
Monoisotopic mass refers to the mass of the most common isotope of each element in a molecule. It is used when extreme precision is needed, such as in determining the structure of complex molecules.
Monoisotopic Isotope Selection
The selection of monoisotopic isotopes involves choosing the most abundant stable isotope for each element. For instance, Carbon-12 is selected for carbon due to its prevalence and stability.
Calculation Methods
Monoisotopic mass is calculated by:
- Identifying the most abundant isotope of each element.
- Summing their masses: Adding the atomic masses of these isotopes gives the monoisotopic mass.
Applications in Science
Use in Mass Spectrometry
In mass spectrometry, the monoisotopic mass helps in accurately identifying molecular ions and fragments, crucial for analyzing complex mixtures and biological samples.
Role in Molecular Identification
Identifying molecules in chemical and biological research heavily relies on knowing their exact monoisotopic mass, enabling scientists to deduce molecular structures and compositions.
Average Mass
Defining Average Mass
Average mass, also known as average atomic mass, represents the weighted average of the isotopic masses of all naturally occurring isotopes of an element, considering their abundance.
Calculation of Average Atomic Mass
The average mass is calculated by:
- Multiplying the mass of each isotope by its relative abundance.
- Summing these products: The result gives a value closer to what is most commonly found in nature.
Concept of Relative Abundance
Relative abundance is the percentage of a particular isotope found naturally compared to all isotopes of that element. It influences the average mass calculation significantly.
Practical Applications
Use in Calculations
Average mass is commonly used in:
- Educational settings: Simplifies teaching basic chemistry concepts.
- Routine chemical calculations: Sufficient for most laboratory and industrial needs.
Significance in Education
Understanding average mass allows students to better grasp concepts such as molecular weight and molar mass, which are foundational in chemistry education. This knowledge bridges their learning to more complex chemical behaviors and reactions.
Comparison
Key Differences
The distinction between monoisotopic mass and average mass lies primarily in their calculation and usage. Monoisotopic mass is the exact mass of the most abundant isotopes of the elements in a molecule, making it critical for high precision tasks like spectrometry. Average mass, however, is a statistical average that considers the weighted isotopic composition of elements as they naturally occur, useful for general calculations and educational purposes.
Calculation Contrast
- Monoisotopic Mass: Calculated using the most abundant isotope for each element.
- Average Mass: Derived by taking a weighted average of all isotopic masses based on their natural abundance.
Impact on Scientific Data
The choice between using monoisotopic or average mass can significantly impact scientific outcomes:
- Precision: Monoisotopic mass is preferred when utmost precision is required, especially in identifying and quantifying substances.
- General Use: Average mass is often adequate for broader, less precise applications such as basic education and routine lab work.
Case Studies
Examples in Research
Studies often utilize monoisotopic mass in fields like proteomics where the exact mass of proteins must be determined to understand biological processes. For instance, in identifying enzymes responsible for disease mechanisms, the monoisotopic mass helps pinpoint the exact molecular structure.
Real-world Application Differences
In pharmaceuticals, monoisotopic mass is crucial for ensuring the precise composition of complex drug molecules, whereas in educational settings, average mass simplifies the introduction of chemical calculations to students.
Importance in Various Fields
Role in Pharmacology
Drug Development and Analysis
- Identification: Monoisotopic mass is essential for identifying active pharmaceutical ingredients by ensuring the accuracy of molecular weights.
- Quality Control: Helps in controlling the quality of synthesized drug compounds by matching their masses to theoretical values.
Precision in Medication Formulation
Precise mass measurements ensure that dosages are accurate and consistent, which is crucial for patient safety and drug efficacy.
Importance in Environmental Science
Tracing Pollution Sources
- Analyzing samples: Average mass calculations are used to estimate the origins of pollutants by comparing isotopic signatures with those of potential sources.
- Monitoring: Helps in monitoring environmental changes over time by observing shifts in isotopic compositions.
Studying Climate Change Effects
Isotopic analysis, including both monoisotopic and average mass measurements, plays a vital role in studying the effects of climate change:
- Carbon Tracking: Differentiating between carbon isotopes helps scientists understand carbon cycling and its sources, whether natural or anthropogenic.
- Environmental Forensics: Helps in forensic investigations to determine the impact of pollutants and trace their origins in the ecosystem.
Frequently Asked Questions
What is monoisotopic mass?
Monoisotopic mass is the mass of a molecule derived from the most abundant isotope of each element it contains. This value is critical in applications requiring high precision like mass spectrometry, where exact mass measurements are necessary for identifying molecular structures.
How is average mass calculated?
Average mass is calculated by taking the weighted mean of the isotopic masses of all atoms in a molecule, based on their occurrence in nature. This calculation involves multiplying the mass of each isotope by its natural abundance, then summing these values to provide a representative figure for that element or compound.
Why are these masses important in science?
Understanding and using both monoisotopic and average masses allow scientists to perform more accurate and meaningful experiments. They are essential in fields such as pharmacology for drug development, in environmental science for tracking pollutant sources, and in chemistry for educational purposes.
Can monoisotopic mass vary between samples?
No, the monoisotopic mass of a molecule remains constant as it is based on the most stable isotopes, which do not change. However, the measured value might vary slightly in experimental settings due to instrumental precision and other technical factors.
Conclusion
The concepts of monoisotopic and average mass provide a foundation for precise scientific work, impacting a range of disciplines from medicine to environmental studies. As such, a clear grasp of these measurements not only enhances the accuracy of scientific research but also enriches the educational approaches in chemistry and related fields.
In sum, whether for advanced research or classroom learning, the precise understanding and application of monoisotopic and average masses play a crucial role in the progress and reliability of scientific endeavors. Their ongoing relevance underscores the importance of detailed and accurate mass measurement in the ever-evolving landscape of science and technology.