Arsenic and phosphorus are two elements that, despite their presence in the periodic table, exhibit distinct characteristics and impacts. Both elements play crucial roles in various applications, ranging from industry to agriculture, yet their properties and effects on health and the environment differ significantly. A clear understanding of these differences is vital for professionals across multiple sectors, as well as for the general public interested in health and environmental safety.
Arsenic, typically notorious for its toxicity, is a metalloid with various applications in alloys, electronics, and wood preservation. On the other hand, phosphorus is a non-metal essential for life, crucial in agriculture as a fertilizer, and used in products like safety matches and fireworks. While arsenic poses significant health risks, phosphorus is necessary for biological functions but can be hazardous in certain forms.
Both elements are found naturally in the environment and have substantial industrial uses that can lead to environmental pollution. The dual nature of arsenic and phosphorus—as beneficial and hazardous—illustrates the complexity of their roles in modern science and industry, making a comprehensive understanding of their differences imperative for effective management and utilization.

Basic Properties
Arsenic Overview
Chemical Characteristics
Arsenic is a metalloid, exhibiting properties of both metals and non-metals, which makes it versatile but also dangerous. It appears in several allotropic forms, though the most common is a brittle, gray metallic form. As an element, arsenic is known for its high electron affinity and excellent conductivity of heat and electricity. These properties make it particularly useful in various industrial applications.
Common Uses and Occurrences
Naturally occurring in the Earth’s crust, arsenic is often found in minerals such as arsenopyrite, realgar, and orpiment. Industrially, arsenic is significant in the production of lead alloys, particularly in car batteries and ammunition. It is also used in the semiconductor industry to produce gallium arsenide, which is essential for integrated circuits and light-emitting diodes (LEDs). Despite its toxicity, arsenic compounds are used as preservatives for wood and in the production of certain pesticides and herbicides.
Phosphorus Overview
Chemical Characteristics
Phosphorus is a non-metal that is essential to life. It normally exists in several allotropic forms: white, red, and black phosphorus, each with unique properties. White phosphorus is highly reactive, whereas red phosphorus is more stable and less reactive. Black phosphorus, the most stable form, has a layered structure and is relatively rare.
Common Uses and Occurrences
Phosphorus is primarily used to manufacture phosphate fertilizers, which are critical for global agriculture. It is also a key component in detergents, where it serves to soften water, and in the manufacture of steel and phosphor bronze. In consumer products, phosphorus compounds are found in toothpaste, where they aid in oral hygiene, and in safety matches, where red phosphorus is used on the striking surface.
Chemical Structures
Arsenic Structure
Atomic Composition
Arsenic has an atomic number of 33, which places it in group 15 of the periodic table. Its electronic configuration is [Ar]3d^10 4s^2 4p^3, showing a half-filled p subshell, which contributes to its stability and reactivity.
Molecular Behavior
In its elemental form, arsenic tends to form covalent bonds. In the environment, it is commonly seen in two oxidation states: +3 and +5. These states play crucial roles in the formation of arsenic compounds with other elements, influencing both the toxicity and behavior of these compounds in biological and environmental systems.
Phosphorus Structure
Variations (White, Red, Black Phosphorus)
- White Phosphorus: Consists of P4 tetrahedra, is highly reactive, and glows in the dark when exposed to oxygen due to slow combustion.
- Red Phosphorus: Obtained by heating white phosphorus in the absence of air at 300°C, used in safety matches, and is much less reactive.
- Black Phosphorus: Has a layered structure, similar to graphite, and exhibits good electrical conductivity.
Atomic and Molecular Formation
The various allotropic forms of phosphorus demonstrate different molecular formations that directly influence their physical and chemical behaviors. These structures determine the reactivity and applications of phosphorus in different industrial and biological contexts.
Health Impacts
Arsenic Toxicity
Exposure Routes
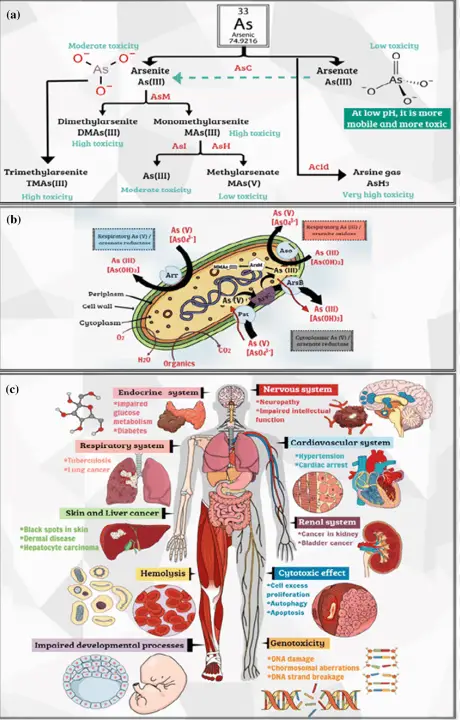
Human exposure to arsenic can occur through various channels, primarily through drinking water contaminated with arsenic, ingestion of foods grown in arsenic-contaminated soil, and inhalation of arsenic-laden dust in industrial settings.
Health Effects
Chronic arsenic exposure is known to cause serious health problems, including skin lesions, cardiovascular diseases, and various forms of cancer. Acute poisoning can lead to gastrointestinal distress, severe neurological effects, and even death. The severity of arsenic’s toxicity underscores the importance of monitoring and controlling exposure.
Phosphorus Benefits
Role in Biological Systems
Phosphorus is critical for cellular function and overall vitality. It forms part of DNA and RNA structures and is essential in energy transfer through ATP, facilitating numerous bodily functions including muscle contraction and nerve conduction.
Health Benefits and Risks
While phosphorus is indispensable for life, excessive intake can lead to detrimental health conditions such as kidney damage and osteoporosis, particularly in individuals with compromised renal function. Conversely, phosphorus deficiency can result in anemia, muscle weakness, and general fatigue, highlighting its critical role and the need to maintain proper balance in its intake.
Environmental Impact
Arsenic in the Environment
Natural and Anthropogenic Sources
Arsenic is naturally present in the Earth’s crust and is released into the environment through volcanic activity and erosion of rocks. Human activities, however, significantly enhance its dispersion through mining, smelting, burning of fossil fuels, and use in agriculture and forestry as pesticides and herbicides. These sources have led to widespread environmental contamination.
Effects on Ecosystems
Arsenic’s presence in the environment can be detrimental to ecosystems. It can reduce plant growth and lead to the death of vegetation by interfering with photosynthesis. In aquatic systems, arsenic contamination affects fish and other aquatic life, leading to reduced biodiversity. Arsenic also bioaccumulates in the food chain, posing significant risks to animal and human health.
Phosphorus in the Environment
Role in Nutrient Cycling
Phosphorus plays a critical role in environmental nutrient cycles, especially in aquatic ecosystems. It is a key nutrient that promotes algae and plant growth. However, its natural cycle is significantly altered by human activities, primarily through the extensive use of phosphorus-based fertilizers in agriculture.
Pollution Concerns
The main concern with phosphorus in the environment is its role in eutrophication, which causes excessive growth of algae in water bodies. This can lead to oxygen depletion in the water, killing fish and other aquatic organisms. The resulting algal blooms can also produce toxins that affect wildlife and human health.
Industrial Uses
Uses of Arsenic
Alloys and Electronics
Arsenic is crucial in the creation of various alloys. It makes lead harder, thus being essential in the manufacturing of car batteries and bullets. In electronics, arsenic is used to produce gallium arsenide, a semiconductor material used in high-speed integrated circuits and infrared light-emitting diodes.
Pesticides and Wood Preservatives
Arsenic compounds have historically been used in pesticides and herbicides due to their effectiveness in pest control. They are also used as wood preservatives to protect against rot and insect damage, significantly extending the life of wood products.
Uses of Phosphorus
Fertilizers and Detergents
Phosphorus is a primary component of phosphate fertilizers, which are crucial for modern agriculture. It is also used in detergents as a water softener to prevent the formation of insoluble mineral deposits.
Fireworks and Safety Matches
In fireworks, phosphorus provides the necessary oxygen supply to produce vibrant colors and effects. Red phosphorus is used in the safety strike panels of matchboxes, offering a friction surface for ignition.
Safety and Handling
Handling Arsenic
Safety Precautions
Handling arsenic requires strict safety measures to prevent poisoning and environmental contamination:
- Use of personal protective equipment (PPE), such as gloves and respirators
- Ensuring proper ventilation in work areas
- Regular monitoring of arsenic levels in the workplace and natural settings
Regulatory Guidelines
Various international and national agencies regulate the use of arsenic to protect human health and the environment. These regulations cover exposure limits, safe disposal, and remediation of contaminated sites.
Handling Phosphorus
Safety Tips
Safety measures for handling phosphorus, especially the more reactive white phosphorus, include:
- Storing under water to prevent contact with air
- Using non-metallic tools to handle phosphorus
- Wearing protective clothing to prevent skin contact
Storage and Disposal
Proper storage and disposal are vital to prevent environmental contamination and ensure safety:
- Phosphorus should be stored in airtight containers under inert conditions
- Disposal should follow local environmental regulations to prevent contamination of water sources
Recent Research
Studies on Arsenic
Innovations in Arsenic Remediation
Recent advances include new methods for removing arsenic from water, such as novel filtration materials and genetic engineering approaches to create bio-remediation systems using plants and bacteria.
Recent Discoveries in Toxicity and Treatment
Ongoing research continues to uncover more about arsenic’s mechanisms of toxicity and its long-term health effects, which guides the development of more effective treatment and management strategies.
Studies on Phosphorus
New Applications and Technologies
Innovations in phosphorus use include developing more efficient fertilizers that reduce environmental impact and exploring its potential in renewable energy technologies.
Sustainability in Phosphorus Use
Research is also focused on sustainable phosphorus management strategies to address the depletion of phosphorus resources and minimize its environmental footprint.
Frequently Asked Questions
What is Arsenic?
Arsenic is a metalloid in the periodic table known for its toxicity. It is commonly found in the environment and used in various industrial processes, particularly in alloys, electronics, and as a wood preservative. Despite its utility, arsenic poses significant health risks, including cancer and skin lesions.
What is Phosphorus?
Phosphorus is a non-metallic element vital to life, primarily used in agriculture as a key component of fertilizers. It also has applications in household products and pyrotechnics. Phosphorus exists in several forms, each with different properties and safety levels.
How do Arsenic and Phosphorus affect human health?
Arsenic is highly toxic and can lead to severe health issues, including cancer, cardiovascular diseases, and diabetes. In contrast, phosphorus is essential for human health, playing a critical role in bone formation, energy storage, and cellular repair, although excessive intake can be harmful.
What are the environmental impacts of Arsenic and Phosphorus?
Arsenic contamination can lead to severe environmental degradation, affecting water sources, soil health, and biodiversity. Phosphorus, while crucial for ecosystems, can contribute to water pollution and eutrophication when mismanaged, leading to harmful algal blooms and deteriorating water quality.
How are Arsenic and Phosphorus used in industries?
Arsenic is used in the production of semiconductors, glass, and pesticides, among other applications. Phosphorus is primarily used to make fertilizers, detergents, and matches, as well as in metallurgy and fireworks.
Conclusion
The exploration of arsenic and phosphorus uncovers a stark dichotomy between their roles and implications. While both elements are indispensable for their respective uses in various industries, their environmental and health impacts necessitate careful handling and regulation. The balancing act between leveraging their benefits and mitigating their risks underscores the importance of ongoing research and technological innovation.
Understanding the distinct characteristics of arsenic and phosphorus is not only crucial for those directly handling these elements but also for the broader public, whose safety and health depend on the responsible management of these substances. As we continue to rely on these elements for numerous applications, their study remains a cornerstone of both industrial and environmental science.

