Colloidal systems, ubiquitous in both nature and industry, encompass a wide range of complex phenomena critical to various scientific and commercial applications. At the heart of this complexity are micelles and colloidal particles, two fundamental components whose differences are as pivotal as their individual characteristics. These small but mighty particles play essential roles, from cleaning agents in household products to carriers in drug delivery systems.
Micelles and colloidal particles, though often discussed together, differ significantly in their structure, formation, and behavior. Micelles are self-assembled structures formed when amphiphilic molecules, like detergents, arrange themselves into spherical forms in aqueous solutions. In contrast, colloidal particles are larger and can consist of various substances, often dispersed throughout another medium, without the specific self-assembly behavior seen in micelles.
The importance of understanding these particles extends beyond academic curiosity; it influences the development of new materials, enhances industrial processes, and leads to innovations in healthcare and environmental management. As such, their study not only broadens scientific knowledge but also facilitates advancements across multiple sectors.
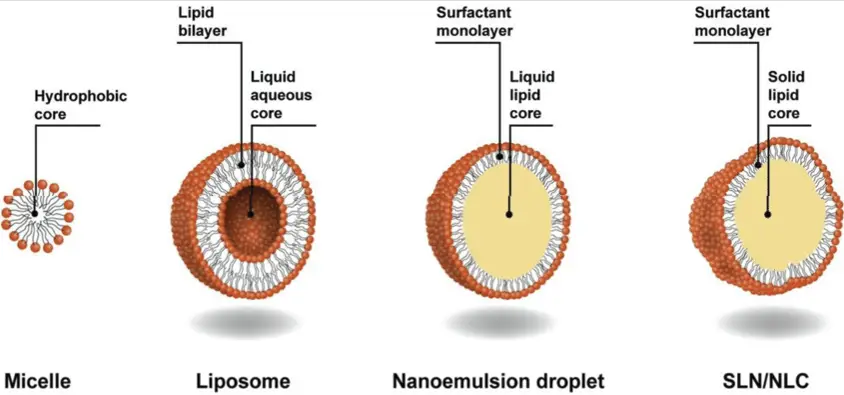
Basics of Colloids
Definition and Characteristics
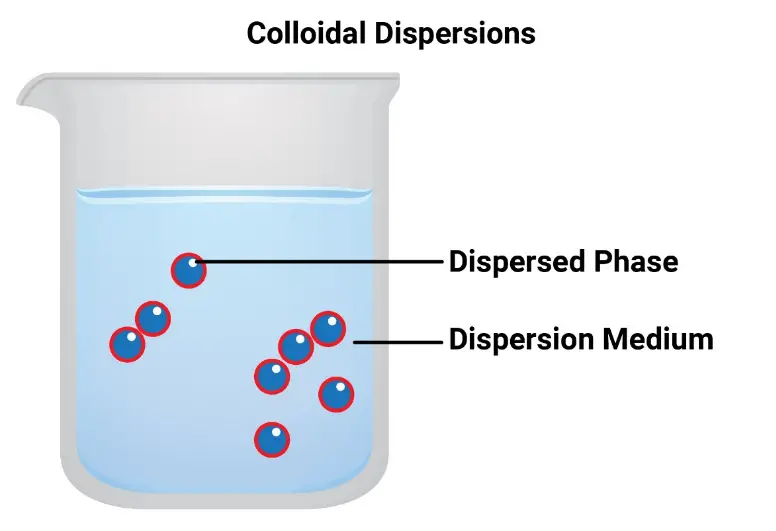
Colloids are mixtures where one substance is dispersed evenly throughout another. The particles in a colloid are larger than those in a solution but smaller than those in a suspension, typically ranging from 1 to 1000 nanometers in diameter. These particles are large enough to scatter light, a phenomenon known as the Tyndall effect, yet too small to settle under gravity, which gives colloidal suspensions their cloudy or opaque appearance.
Types of Colloidal Systems
Colloidal systems can be categorized based on the phases of the dispersed particles and the medium. The main types include:
- Sol: Solid particles in a liquid
- Gel: Liquid particles in a solid
- Emulsion: Liquid droplets in another liquid
- Foam: Gas bubbles in a liquid
- Aerosol: Solid or liquid particles in a gas
Each type plays a critical role in various applications, from medical formulations to food products.
Common Examples in Everyday Life
Everyday examples of colloids include milk (an emulsion of fat in water), whipped cream (a foam of air in cream), and smoke (an aerosol of carbon particles in air). These examples highlight the ubiquity of colloids in daily life and their importance in food science, environmental science, and technology.
Introduction to Micelles
Definition of Micelles
Micelles are a specific type of colloid formed when amphiphilic molecules, such as soaps and detergents, organize themselves into spherical structures in a liquid. The molecules arrange with their hydrophobic (water-repelling) tails inward and their hydrophilic (water-attracting) heads outward, creating a structure that can encapsulate oil-based dirt particles.
Formation Process
The formation of micelles occurs when the concentration of amphiphilic molecules in a solution reaches the critical micelle concentration (CMC). Beyond this point, the molecules spontaneously form micelles to minimize the free energy of the system. This process is crucial in many biological and chemical processes.
Role in Biological Systems and Industry
In biological systems, micelles facilitate the absorption of fat-soluble vitamins and regulate the transport of lipids across cell membranes. Industrially, micelles are vital in applications such as drug delivery systems, where they can encapsulate hydrophobic drugs, improving their solubility and bioavailability.
Colloidal Particles Explained
Characteristics of Colloidal Particles
Colloidal particles possess unique characteristics that differentiate them from other materials. They exhibit Brownian motion, where the particles move randomly due to collisions with solvent molecules, and they are stable against settling under normal conditions. These properties make colloids essential in scientific and industrial applications.
How They Differ from Solutions and Suspensions
The primary difference between colloidal particles and those in solutions and suspensions lies in their size. Colloidal particles are larger than those in true solutions but smaller than those in suspensions. This size difference affects their stability and behavior, as colloidal particles do not settle out or separate on standing, unlike suspensions.
Applications in Various Fields
Colloidal particles find applications across a broad spectrum of fields:
- Medicine: Targeted drug delivery and diagnostic imaging
- Cosmetics: Emulsions for creams and lotions
- Food: Stabilizers and thickeners
- Environmental Science: Water purification and soil remediation
These applications demonstrate the versatility of colloidal particles and their capacity to enhance product performance and efficacy in diverse industries.
Comparative Analysis
Structural Differences
Micelles and colloidal particles differ fundamentally in their structure. Micelles are characterized by a distinct core-shell structure due to the amphiphilic nature of their constituent molecules. These structures are dynamic and can change based on environmental conditions such as pH, temperature, and ionic strength. On the other hand, colloidal particles may be composed of a single substance or a combination but do not necessarily have the core-shell architecture seen in micelles.
Size and Scale Comparison
The size of micelles typically ranges from 4 to 100 nanometers, depending on the type of surfactant and conditions. In contrast, colloidal particles have a broader size spectrum and can be up to 1000 nanometers. This size difference is crucial for applications that rely on size-dependent properties, such as drug delivery and catalysis.
Stability Factors
Stability is a key difference; micelles are stable due to the energetic favorability of their formation when the critical micelle concentration is reached. Colloidal particles require stabilizing agents to prevent aggregation. Factors affecting their stability include particle size, zeta potential (charge), and the presence of stabilizing agents.
Interaction with Light
How Micelles Interact with Light
Micelles can affect light through scattering and absorption, influencing their use in applications like drug delivery and diagnostics. The arrangement of molecules in micelles can encapsulate substances that either absorb or scatter light, making them useful in therapeutic and imaging techniques.
Colloidal Particle Light Interactions
Colloidal particles scatter light, which is the principle behind the Tyndall effect. This scattering is significant in determining the stability and size distribution of colloidal systems through techniques like dynamic light scattering.
Practical Implications of These Interactions
The interaction with light has practical applications in:
- Medical imaging: Enhancing contrast in techniques such as MRI and ultrasound.
- Quality control: Monitoring the stability and quality of colloidal products.
Surfactant Role
Role of Surfactants in Micelle Formation
Surfactants are crucial for micelle formation. They reduce the surface tension between the liquid and the air or between different liquids, facilitating the self-assembly of micelles. This process occurs when the surfactant concentration exceeds the critical micelle concentration.
Impact on Colloidal Stability
In colloidal systems, surfactants can act as stabilizers by adsorbing to the surface of colloidal particles and preventing them from aggregating. The choice of surfactant, its concentration, and the nature of the colloid are critical for optimizing stability.
Differences in Surfactant Concentration Effects
The effects of surfactant concentration are distinctly different between micelles and colloids. Exceeding the critical micelle concentration is necessary for micelle formation, while in colloidal systems, the right concentration of surfactants ensures stability without causing excess solubility or aggregation.
Applications in Industry
Micelles in Pharmaceuticals
Micelles are used to improve the solubility of hydrophobic drugs, enhancing their delivery and efficacy. They are integral in formulations where active ingredients must be protected until they reach specific sites within the body.
Colloidal Particles in Manufacturing
In manufacturing, colloidal particles are used in the production of coatings, ceramics, and even electronics, where their unique properties enable precise control over product characteristics.
Innovative Uses in Technology and Medicine
Innovations include:
- Targeted drug delivery systems using micelles that respond to specific stimuli.
- Advanced materials for improved sensors and devices.
Environmental Impact
Biodegradability of Micelles
Micelles formed from biodegradable surfactants offer an environmentally friendly option, reducing the impact of chemical residues.
Colloidal Particles in Environmental Systems
Colloidal particles can be designed to remove pollutants from water or soil, playing a crucial role in environmental cleanup efforts.
Sustainability Considerations
Sustainable practices in the production and use of micelles and colloidal particles are essential to minimize ecological footprints and promote environmental health.
Research Trends
Recent Advances in Colloidal Science
Recent research focuses on synthesizing colloidal particles with tailored properties for specific applications, such as in optoelectronics and therapeutic agents.
Innovations in Micelle Applications
Innovations include development of responsive micelles that can change their structure in response to environmental triggers, enhancing their functionality in drug delivery.
Future Prospects in Research and Industry
The future holds promising advancements in the synthesis and application of micelles and colloidal particles, driven by ongoing research and the increasing demand for sophisticated materials and delivery systems.
Frequently Asked Questions
What are micelles?
Micelles are molecular assemblies formed when amphiphilic molecules, such as soaps and detergents, organize into spherical structures in a liquid. These structures are typically composed of a hydrophobic (water-repelling) core and a hydrophilic (water-attracting) outer layer, allowing them to solubilize oily substances in water.
How do colloidal particles differ from true solutions?
Colloidal particles are larger than molecules found in true solutions and are dispersed throughout a medium where they remain suspended. Unlike solutions, where solutes dissolve, colloidal particles are distinct in that they do not dissolve but remain distributed, which affects the properties of the colloid, like scattering light.
Can micelles exist in non-aqueous solutions?
Yes, micelles can form in non-aqueous solutions if the solvent can appropriately interact with the amphiphilic molecules, similar to how water interacts. Solvents like alcohols and certain oils can support micelle formation, depending on the nature of the amphiphile and the conditions of the environment.
What role do surfactants play in the formation of micelles?
Surfactants are crucial for micelle formation as their amphiphilic nature allows them to reduce surface tension between two liquids or a liquid and a solid. This characteristic facilitates the self-assembly of surfactant molecules into micelles once the concentration exceeds a certain threshold known as the critical micelle concentration (CMC).
Conclusion
The distinctions between micelles and colloidal particles offer a glimpse into the intricate world of colloidal chemistry, where size, structure, and environmental interactions dictate functionality. These differences are not merely academic; they have practical implications in designing more effective drugs, creating better cleaning agents, and innovating in material science. Recognizing and leveraging these variations can lead to significant technological and scientific advancements.
In conclusion, the exploration of micelles and colloidal particles not only enriches our understanding of chemical and physical phenomena but also paves the way for future innovations. As research continues to unravel the complex behaviors of these particles, their full potential can be harnessed, influencing a wide array of industries and improving everyday products.

