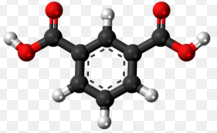
Isophthalic acid and terephthalic acid are aromatic dicarboxylic acids pivotal in the synthesis of numerous industrial products. Each compound possesses a unique chemical structure that defines its role and utility in various applications, from plastics to resins. Despite their similar molecular frameworks, the differences in their chemical properties and uses are significant, influencing industries worldwide.
Isophthalic acid and terephthalic acid differ primarily in their molecular structure orientations and resultant physical properties. Isophthalic acid, with a meta orientation of the carboxyl groups, offers flexibility and improved impact resistance in polymers. In contrast, terephthalic acid, featuring a para-oriented configuration, provides enhanced strength and stiffness, making it ideal for rigid polymer applications.
These acids are not just foundational in producing everyday plastic and polyester products but also play crucial roles in high-performance materials used in automotive and aerospace industries. Their distinct characteristics help in tailoring materials for specific functional requirements, making them indispensable in modern manufacturing processes.
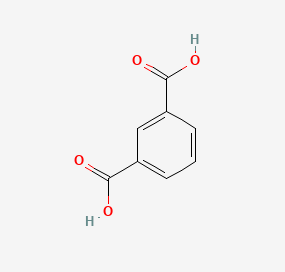
Chemical Structures
Basic Molecular Structure Comparison
Isophthalic acid and terephthalic acid, while both aromatic dicarboxylic acids, have distinct differences in their molecular structure. Isophthalic acid is composed of a benzene ring with two carboxylic acid groups (COOH) attached at the 1 and 3 positions (meta position). In contrast, terephthalic acid has its carboxylic acid groups attached at the 1 and 4 positions (para position) of the benzene ring. This subtle shift in the placement of functional groups leads to significant differences in their physical and chemical properties.
Functional Groups and Orientation
The orientation of the carboxyl groups significantly affects the properties of these acids. In isophthalic acid, the meta position allows for more flexibility in the molecular structure, which is crucial for certain applications such as plasticizers and unsaturated polyester resins. Terephthalic acid’s para-oriented carboxyl groups create a more linear and rigid structure, ideal for the production of strong, stiff fibers like those used in PET (polyethylene terephthalate) production.
Production Methods
Isophthalic Acid Synthesis
The production of isophthalic acid typically involves the catalytic oxidation of m-xylene using air in the presence of a cobalt-manganese catalyst. The process is carefully controlled to optimize yield and purity, crucial for its use in high-performance applications. The steps include:
- Mixture Preparation: Combining m-xylene with acetic acid and catalysts.
- Oxidation: Air is bubbled through the mixture to oxidize the m-xylene to isophthalic acid.
- Purification: The crude product is refined to achieve the desired purity levels for industrial use.
Terephthalic Acid Production
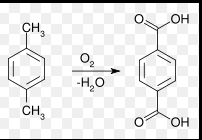
Terephthalic acid is predominantly produced by the catalytic air oxidation of p-xylene. This process is more complex due to the need for high purity in the final product, which is essential for polyester synthesis. Key steps are:
- Reaction Setup: p-xylene is mixed with a solvent (usually acetic acid) along with catalysts.
- Oxidation Process: Air or oxygen is used to oxidize p-xylene at high temperatures.
- Recovery and Purification: The acid is recovered from the reaction mixture and purified through hydrogenation and crystallization to remove any impurities.
Physical Properties
Melting Points and Solubility
Isophthalic acid has a lower melting point (around 348°F or 175°C) compared to terephthalic acid, which melts at about 428°F (220°C). Solubility in water for both compounds is quite low, but they are readily soluble in organic solvents such as acetone, ethanol, and benzene. This solubility profile is crucial for their application in polymer chemistry.
Appearance and State
At room temperature, both acids typically appear as white crystalline solids. Their physical state makes them suitable for easy handling and processing in various chemical synthesis applications.
Chemical Properties
Reactivity with Other Chemicals
Both isophthalic and terephthalic acids show high reactivity with alcohols, forming corresponding esters through esterification reactions. These reactions are fundamental in producing polyesters, where the acids react with glycols such as ethylene glycol.
Stability Under Various Conditions
Isophthalic acid exhibits good thermal stability and excellent resistance to light and water, making it suitable for outdoor applications. Terephthalic acid also shows stability under high temperatures, though its rigid structure makes it less resistant to impact compared to isophthalic acid. Both compounds are stable under normal storage conditions but require careful handling due to their corrosive nature when in contact with skin or mucous membranes.
Industrial Uses
Role in Polyester Production
Isophthalic acid and terephthalic acid are crucial in the production of polyester fibers and resins, which are widely used in textiles, packaging, and high-performance materials. Polyester production involves the polymerization of these acids with glycols such as ethylene glycol.
- Isophthalic Acid: Primarily used to enhance the properties of polyesters, making them more suitable for soft furnishings and films due to their flexibility and durability.
- Terephthalic Acid: Essential in the manufacture of polyethylene terephthalate (PET), used in clothing, bottles, and food packaging. PET is favored for its strength, thermal stability, and clarity.
Applications in Resin Manufacturing
Beyond polyesters, these acids find applications in resin manufacturing, where their properties help produce varnishes, coatings, and gel coats.
- Isophthalic Acid Resins: Known for their excellent water resistance and mechanical properties, making them ideal for marine applications, including boat hulls and decks.
- Terephthalic Acid Resins: Used in making high-gloss coatings that require a high degree of rigidity and thermal resistance, common in automotive and appliance applications.
Environmental Impact
Biodegradability and Toxicity
Both isophthalic and terephthalic acids pose environmental challenges. They are not readily biodegradable, which means they can persist in the environment.
- Isophthalic Acid: Low toxicity but can cause ecological damage if released in large quantities.
- Terephthalic Acid: Considered low in toxicity for humans but can be harmful to aquatic life if not managed properly.
Impact on Wastewater Treatment
The production and use of these acids generate wastewater that requires treatment to remove any residual chemical content before discharge.
- Treatment Techniques: Methods include neutralization, biological treatment, and advanced oxidation processes. These techniques help mitigate the impact of these acids on water bodies.
Economic Aspects
Global Market Trends
The global market for isophthalic and terephthalic acid is driven by the demand for polyesters and resins.
- Market Growth: Driven by the expanding textile and packaging industries, especially in Asia-Pacific regions where manufacturing growth is robust.
- Innovation and Development: Advances in chemical processing and recycling technologies are shaping the market dynamics.
Pricing and Consumption Patterns
- Pricing Fluctuations: Prices for these acids are influenced by the volatility in raw material costs, particularly benzene and xylene derivatives.
- Consumption Trends: High demand in developing regions is contrasted with mature markets focusing on sustainability and recycling initiatives.
Health and Safety
Handling Guidelines
Proper handling and storage of isophthalic and terephthalic acids are crucial to ensure safety in industrial settings.
- Storage Conditions: Store in cool, well-ventilated areas away from moisture.
- Handling Protocols: Use appropriate personal protective equipment (PPE) such as gloves and eye protection when handling these chemicals.
Exposure Risks and Protective Measures
- Inhalation and Contact Risks: Exposure can lead to respiratory issues and skin irritation. Ensuring good industrial hygiene and ventilation reduces these risks.
- Safety Training: Regular training for workers on handling procedures and emergency response is essential to maintain a safe working environment.
Frequently Asked Questions
What is Isophthalic Acid?
Isophthalic acid is an aromatic dicarboxylic acid, characterized by its meta-positioned carboxyl groups on the benzene ring. This configuration imparts unique properties that are crucial for producing high-quality, durable polyester fibers and resins used in a variety of industrial applications.
How is Terephthalic Acid Produced?
Terephthalic acid is primarily produced through the catalytic oxidation of p-xylene in acetic acid, in the presence of air. This method facilitates the creation of a highly pure form of terephthalic acid, essential for synthesizing polyethylene terephthalate (PET) used in textiles and packaging.
Why are These Acids Important?
Isophthalic acid and terephthalic acid are fundamental to the chemical industry due to their roles in creating polyesters and resins. These materials are pivotal in a range of applications, from clothing and packaging to automotive and aerospace components, highlighting their industrial significance.
Can These Acids be Recycled?
Both isophthalic acid and terephthalic acid are integral to the production of recyclable polymers such as PET. The recycling processes help in reducing environmental impact and promoting sustainable use of resources in manufacturing.
Conclusion
The nuanced differences between isophthalic acid and terephthalic acid underscore their unique roles in industrial applications. Each acid’s specific molecular structure and properties dictate its suitability for various products, making them critical to advancements in materials science. As industries continue to evolve, the demand for specialized materials like these will likely increase, reflecting their ongoing importance.
The future of industrial applications continues to lean heavily on the development of materials that can offer both sustainability and performance. In this light, isophthalic acid and terephthalic acid are more than just chemical compounds; they are the building blocks of future innovations in material science, driving towards more efficient, durable, and environmentally friendly products.