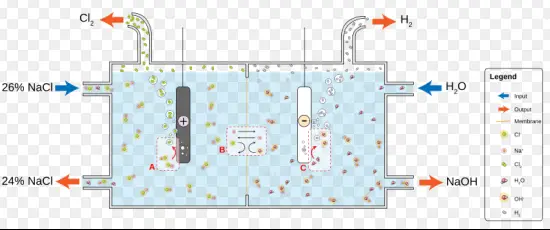
Conduction is a fundamental concept in both physics and engineering, influencing everything from electronic devices to energy systems. At its core, conduction is the process by which heat or electricity is directly transmitted through a material. This phenomenon is critical in the development of technologies and in the understanding of various scientific principles. Two primary forms of conduction are electronic and ionic, each playing distinct roles in modern science and industry.
Electronic conduction occurs when electrons move through a conductor, such as metals, due to a difference in electric potential. Ionic conduction, on the other hand, involves the movement of ions within an electrolyte or in molten salts. The choice between these two types of conduction depends on their respective efficiencies and applications, with electronic conduction being prevalent in solid state devices and ionic conduction often favored in electrochemical applications like batteries and fuel cells.
While both forms of conduction are pivotal for numerous applications, they differ fundamentally in their mechanisms and implications. Electronic conduction is typically associated with solid, crystalline materials where electrons are the primary charge carriers. Ionic conduction, however, is more associated with liquid or semi-solid states where free ions facilitate charge transfer. Understanding these differences is crucial for selecting appropriate materials and methods in various technological and scientific contexts.
Basic Concepts
What is Conduction?
Conduction is the transfer of energy through a medium, typically involving heat or electricity, without any movement of the material as a whole. This process is fundamental in various scientific and technological applications, where understanding how energy is transmitted through materials is crucial for innovation and development.
Types of Conduction
There are mainly two types of conduction: electronic and ionic. Each type plays a critical role in different technological and scientific fields, based on how they transfer energy:
- Electronic Conduction: Involves the movement of electrons.
- Ionic Conduction: Involves the movement of ions.
Electronic Conduction
Definition and Mechanism
Electronic conduction occurs when electrons move across a material, facilitating the transfer of electric current. This process is predominantly observed in metals and semiconductors, where loosely bound electrons are available to move freely in response to an electric field.
Materials and Applications
Materials: Common materials that exhibit excellent electronic conduction include:
- Copper
- Silver
- Aluminum
These materials are characterized by their metallic bonds, which allow electrons to move freely across the atomic lattice.
Applications: Electronic conduction is essential in:
- Electrical wiring
- Circuit boards
- Electronic components
These applications leverage the ability of electrons to carry energy across extensive networks without significant loss.
Ionic Conduction
Definition and Mechanism
Ionic conduction refers to the movement of ions within a material, usually in an electrolyte, where ions are free to move. Unlike electrons, ions are atoms or molecules with a net electric charge, and their movement contributes to the conduction of electricity particularly in aqueous or molten states.
Materials and Applications
Materials: Ionic conduction is commonly observed in:
- Salts
- Acids
- Bases
When these materials dissolve in water or melt, they dissociate into charged particles that can move freely, conducting electricity.
Applications: Ionic conduction is crucial in:
- Batteries
- Fuel cells
- Electrolysis processes
These applications rely on the transfer of ions to facilitate energy storage and conversion.
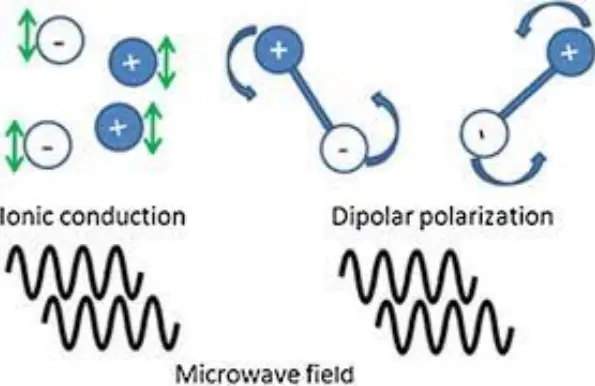
Key Differences
Conductivity Mechanisms
The fundamental difference in conductivity mechanisms between electronic and ionic conduction lies in their charge carriers:
- Electronic Conduction: Electrons act as charge carriers.
- Ionic Conduction: Charged ions (cations and anions) act as charge carriers.
Material Types
The choice of materials also significantly differs:
- Electronic Conduction: Requires materials with free electrons, typically metals.
- Ionic Conduction: Requires electrolytes that can release ions, such as salts in solution.
Usage in Devices
The use of these conduction types in devices depends on the required efficiency and application:
- Electronic Devices: Utilize electronic conduction for rapid and efficient transfer of electricity.
- Electrochemical Devices: Prefer ionic conduction for processes that involve chemical reactions, like battery operation or electrolysis.
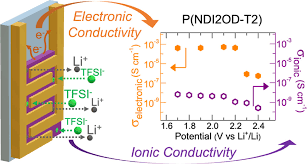
Advantages of Each
Benefits of Electronic Conduction
Electronic conduction offers several key advantages that make it ideal for a variety of applications:
- High Conductivity: Metals like copper and silver are among the best conductors of electricity, facilitating rapid and efficient current transfer with minimal energy loss.
- Reliability: Electronic conduction through metals is highly reliable under a range of environmental conditions, making it suitable for critical applications in industrial and residential settings.
- Durability: Metallic conductors are durable and able to withstand physical stress and high temperatures, which is essential for their use in building infrastructure and manufacturing.
Benefits of Ionic Conduction
Ionic conduction, while different in mechanism, also brings unique advantages:
- Selective Conductivity: Ionic materials can be engineered to selectively conduct specific ions, which is crucial in applications like batteries and sensors.
- Low-Temperature Operation: Unlike metals, ionic conductors can function effectively at lower temperatures, which is beneficial in cold environments and for applications requiring minimal thermal footprints.
- Chemical Reactions: Ionic conduction is essential in processes that involve chemical reactions, such as electrolysis, where the movement of ions is necessary to facilitate these reactions.
Practical Applications
Electronic Conduction Uses
Electronic conduction finds its use in a multitude of applications that are integral to modern life:
- Consumer Electronics: Everything from smartphones to laptops relies on electronic conduction for internal wiring and circuitry.
- Power Transmission: High-voltage power lines utilize materials with high electronic conductivity to efficiently transport electricity over long distances.
- Automotive Systems: Modern vehicles use electronic components extensively for various functions, including engine management and infotainment systems.
Ionic Conduction Uses
Ionic conduction plays a crucial role in many specialized applications:
- Batteries: The operation of batteries, including lithium-ion types, relies on the movement of ions between the anode and cathode to store and release energy.
- Fuel Cells: Ionic conductors enable the migration of hydrogen ions through the electrolyte in fuel cells, generating electricity from chemical energy.
- Water Purification: Electrolysis processes in water purification involve ionic conduction to remove contaminants and soften water.
Challenges and Solutions
Common Challenges
Both types of conduction face specific challenges:
- Corrosion in Metals: Electronic conductors, especially metals, are prone to corrosion over time, which can degrade their performance.
- Ion Transport Efficiency: Ionic conduction can be less efficient due to the larger size and slower movement of ions compared to electrons.
Innovations and Improvements
Ongoing research and technological advancements are addressing these challenges:
- Corrosion-resistant Coatings: Scientists are developing advanced coatings that protect metals from corrosion without impairing their conductive properties.
- Advanced Electrolytes: New materials for electrolytes are being designed to improve the efficiency and speed of ion transport in applications like batteries and fuel cells.
Future Trends
Research Directions
The future of conduction technology is being shaped by exciting research directions:
- Superconductors: Research into materials that can conduct electricity without resistance at higher temperatures could revolutionize power distribution.
- Nanostructured Materials: Nanoengineering materials for better ionic conductivity is a promising field that could enhance the performance of electrochemical devices.
Technological Advancements
Technological advancements continue to push the boundaries of what is possible with both electronic and ionic conduction:
- Flexible Electronics: Developments in flexible conductive materials are enabling new applications in wearable technology and foldable devices.
- Solid-state Batteries: Innovations in solid electrolytes are leading to batteries that are safer and more efficient, with higher energy densities than traditional liquid electrolyte-based systems.
Frequently Asked Questions
What is electronic conduction?
Electronic conduction refers to the transfer of electrical current or heat through the movement of electrons within a material. This type of conduction is most common in metals and semiconductors where electrons are free to move across atoms under the influence of an electric field.
How does ionic conduction work?
Ionic conduction involves the movement of ions instead of electrons. It typically occurs in electrolytes, ionic solutions, or molten salts, where the electrical current results from the flow of positively and negatively charged ions in opposite directions.
Why are different materials used for electronic and ionic conduction?
The choice of materials for specific types of conduction depends on their inherent properties. Metals, with their delocalized electrons, are ideal for electronic conduction. In contrast, materials that can dissolve into ions, like electrolytes, are necessary for ionic conduction.
What are the applications of electronic and ionic conduction?
Electronic conduction is pivotal in the operation of electronic devices such as computers and mobile phones, while ionic conduction is essential in applications involving energy storage and conversion, including batteries and fuel cells.
Conclusion
The distinction between electronic and ionic conduction encapsulates one of the many fascinating aspects of material science. By understanding the fundamental differences in how these types of conduction occur and the materials involved, researchers and engineers can better tailor technologies to suit specific applications. This knowledge not only enhances the efficiency of existing devices but also paves the way for innovative solutions to energy and technology challenges.
As we continue to advance technologically, the roles of electronic and ionic conduction are likely to evolve further, underpinning new breakthroughs and applications. Their study remains a dynamic and crucial field within both scientific research and industrial application, reflecting their broad impact and utility.