Isomerization and hydroisomerization are pivotal chemical processes used extensively across various industries, from pharmaceuticals to petrochemicals. Each process, while similar in its aim to rearrange molecules, employs distinct methods and catalysts to achieve desired chemical structures. Understanding these processes is crucial for professionals involved in chemical manufacturing and process optimization.
Isomerization involves the rearrangement of atoms within a molecule to form isomers, which are compounds with the same molecular formula but different structural configurations. Hydroisomerization builds upon this by incorporating hydrogen into the process, which not only rearranges the molecular structure but also adds hydrogen atoms, thereby saturating the molecule. This results in more stable compounds that are often more useful in industrial applications.
Both processes are employed to enhance the performance and efficiency of chemical products. Isomerization is critical in the production of high-octane gasoline, while hydroisomerization is key to improving the quality of diesel fuels and lubricating oils. The differences in catalysts and conditions between these processes directly impact their applications and the effectiveness of the outcomes.
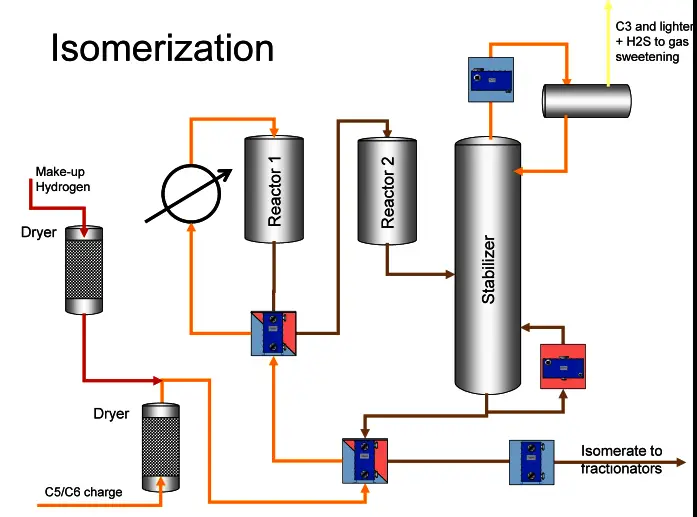
Basic Concepts
Isomerization Overview
Definition and Process
Isomerization is a chemical reaction where molecules transform into different structural forms called isomers. These isomers have the same molecular formula but differ in their chemical structure, resulting in varied physical properties and chemical behaviors. The process involves the rearrangement of carbon skeletons in hydrocarbons and can occur under the influence of catalysts at specific temperatures and pressures.
Types of Isomerism
Isomerism manifests in several forms:
- Structural Isomerism: Also known as constitutional isomerism, this occurs when molecules with the same molecular formula have different bonding patterns and atomic organization.
- Stereoisomerism: This type involves the same molecular formula and sequence of bonded atoms, but with a different three-dimensional orientation. It includes cis-trans isomerism and enantiomerism.
Hydroisomerization Overview
Definition and Process
Hydroisomerization combines the principles of isomerization with hydrogen addition. It involves the rearrangement of molecular structures along with the saturation of the molecule using hydrogen. This not only alters the structure but also increases the molecular stability and reduces the degree of unsaturation.
Comparison with Isomerization
While both processes aim to convert molecules into more useful forms, hydroisomerization typically results in products that are more saturated, making them more stable and less reactive. This is particularly advantageous in fuel industries where stability is crucial.
Chemical Mechanics
Isomerization Mechanics
Key Catalysts Involved
Catalysts play a pivotal role in isomerization. Common catalysts include:
- Acids: Such as sulfuric acid or phosphoric acid, used in alkylation reactions in refineries.
- Metals: Platinum or palladium on an alumina or zeolite base, especially in the petrochemical industry.
Reaction Conditions
Optimal conditions for isomerization generally involve:
- Temperature: Typically ranges from 100°C to 300°C.
- Pressure: Moderate pressures are sufficient to achieve effective isomerization.
Hydroisomerization Mechanics
Role of Hydrogen
Hydrogen in hydroisomerization not only helps in stabilizing the reactive intermediates but also saturates the molecule, reducing unsaturation and preventing the formation of by-products like gums and resins in fuels.
Catalysts and Conditions
Catalysts typically used include noble metals supported on acid sites of zeolites. Conditions are slightly more stringent:
- Temperature: Usually higher than in simple isomerization, up to 350°C.
- Pressure: Higher pressures are required to maintain hydrogen solubility in the reaction mixture.
Industrial Applications
Isomerization Uses
Role in Refining Processes
Isomerization enhances the octane rating of light naphtha within petroleum refining, turning it into high-octane gasoline. This is crucial for meeting the fuel standards and performance specifications of modern engines.
Examples in Everyday Products
Beyond fuels, isomerization processes are used in the production of various plastics and pharmaceuticals, altering properties such as melting point, durability, and efficacy.
Hydroisomerization Uses
Enhanced Product Characteristics
Hydroisomerization is used to improve the quality of diesel fuels and lubricating oils, resulting in products that perform better under a wider range of temperatures and conditions.
Specific Industry Examples
This process is integral in the production of low-sulfur diesel fuels, which are essential for reducing harmful emissions. Hydroisomerization helps achieve the stringent environmental standards set for vehicular emissions.
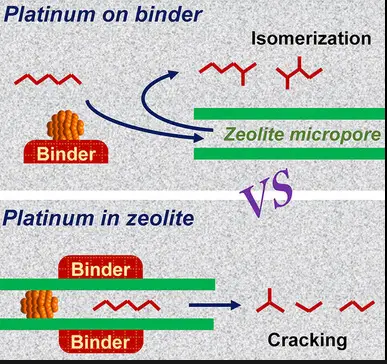
Advantages and Limitations
Isomerization Benefits
Economic Aspects
Isomerization processes significantly contribute to economic efficiency in the chemical and fuel industries. By transforming less valuable straight-chain hydrocarbons into higher-value branched isomers, refineries can produce fuels with higher octane ratings, which are much more valuable in the marketplace. This transformation reduces the need for expensive additives and enables refineries to meet market demands with less raw material.
Environmental Impact
From an environmental perspective, isomerization helps produce fuels that burn cleaner, reducing emissions of harmful pollutants such as nitrogen oxides, unburned hydrocarbons, and carbon monoxide. This aligns with global standards for environmental protection and helps companies avoid hefty fines from non-compliance with environmental regulations.
Hydroisomerization Benefits
Product Yield Improvement
Hydroisomerization increases the yield of usable products from crude oil feedstocks by improving the conversion of heavy hydrocarbons into lighter, more valuable products. This not only maximizes output but also enhances the efficiency of the refining process, leading to significant cost savings and reduced waste.
Environmental Considerations
Hydroisomerization also plays a critical role in producing low-sulfur fuel products, crucial for reducing sulfur dioxide emissions upon combustion. This has significant environmental benefits, contributing to cleaner air and reducing acid rain.
Challenges
Operational Challenges
The complexity of the isomerization and hydroisomerization processes often requires sophisticated equipment and high maintenance costs. Catalyst deactivation, a common issue, necessitates periodic replacement and can disrupt production, leading to increased operational costs.
Comparison of Limitations
While isomerization is less complex, it does not always produce results suitable for all types of feedstocks, as it is less effective with heavier hydrocarbons. Hydroisomerization, though more versatile with heavier feedstocks, requires higher pressures and temperatures, leading to greater energy consumption and operational costs.
Process Optimization
Enhancing Isomerization
Techniques and Strategies
- Catalyst Improvement: Developing more effective catalysts that can operate at lower temperatures and pressures can reduce energy consumption and extend catalyst life.
- Process Control Optimization: Utilizing advanced control systems can optimize the reaction conditions in real-time, improving yield and reducing by-product formation.
Enhancing Hydroisomerization
Technological Advancements
- Catalyst Regeneration Technology: New technologies that allow for the in-situ regeneration of catalysts can help maintain catalyst activity and extend operational periods without shutdowns.
- High-Pressure Technology: Innovations in high-pressure technology can improve the efficiency of hydroisomerization processes, allowing for lower temperatures and reduced energy usage.
Future Perspectives
Emerging Trends in Chemical Processes
The chemical industry is seeing a shift towards more sustainable and energy-efficient processes. Innovations such as biocatalysis and the use of renewable feedstocks are becoming more prevalent. These methods not only enhance the green credentials of chemical processes but also offer new pathways for isomerization and hydroisomerization that are less reliant on non-renewable resources.
Research and Development Directions
Research is increasingly focused on molecular management—designing catalysts at the molecular level to achieve highly specific reactions with minimal unwanted by-products. This precision catalysis could revolutionize how isomerization and hydroisomerization are conducted, leading to even greater efficiencies and reduced environmental impacts.
Frequently Asked Questions
What is Isomerization?
Isomerization is a chemical reaction where molecules are rearranged to form different structural isomers. These isomers have the same molecular formula but differ in physical and chemical properties, which is critical in enhancing fuel quality and pharmaceutical synthesis.
How does Hydroisomerization differ?
Hydroisomerization differs from simple isomerization by the addition of hydrogen into the reaction. This not only changes the molecular structure but also saturates the molecule, leading to increased stability and a higher quality of the end product, particularly in fuel industries.
Why are these processes important?
These processes are fundamental in the production of cleaner, more efficient fuels. They help in converting straight-chain hydrocarbons into branched-chain forms, which burn more efficiently and produce fewer emissions. In pharmaceuticals, they are used to create active forms of various drugs.
Can isomerization occur naturally?
Yes, isomerization can occur naturally under certain conditions, often influenced by light, heat, or specific catalysts found in nature. However, industrial applications typically require controlled environments to ensure efficiency and yield.
Conclusion
Isomerization and hydroisomerization play crucial roles in modern industrial applications by enhancing the properties of chemical compounds. Their significance extends beyond just chemical theory, influencing the practical production of everyday products from gasoline to pharmaceuticals.
The future developments in these processes will likely focus on increasing efficiency, reducing environmental impact, and expanding the range of applicable compounds. Such advancements will continue to support the growing demands of various industries while aligning with global sustainability goals.