Chemistry, at its core, involves understanding how substances interact and transform. Key to these interactions are the concepts of gram equivalent and equivalent weight—terms that are foundational yet often cause confusion. These concepts allow chemists to predict the outcomes of reactions and create precise formulations.
Equivalent weight is essentially the mass of a substance that combines with or displaces 1.008 grams of hydrogen, 8.0 grams of oxygen, or an equivalent weight of another element or compound. Gram equivalent, on the other hand, is the measure of the reactive capacity of a molecule, expressed in grams. It is calculated by dividing the substance’s mass by its equivalent weight.
These terms are crucial for quantifying reaction participants in chemical equations, particularly in processes like titration. Understanding their differences and applications helps improve the accuracy and efficiency of chemical experiments and industrial processes.
Basics of Equivalent Weight
Definition of Equivalent Weight
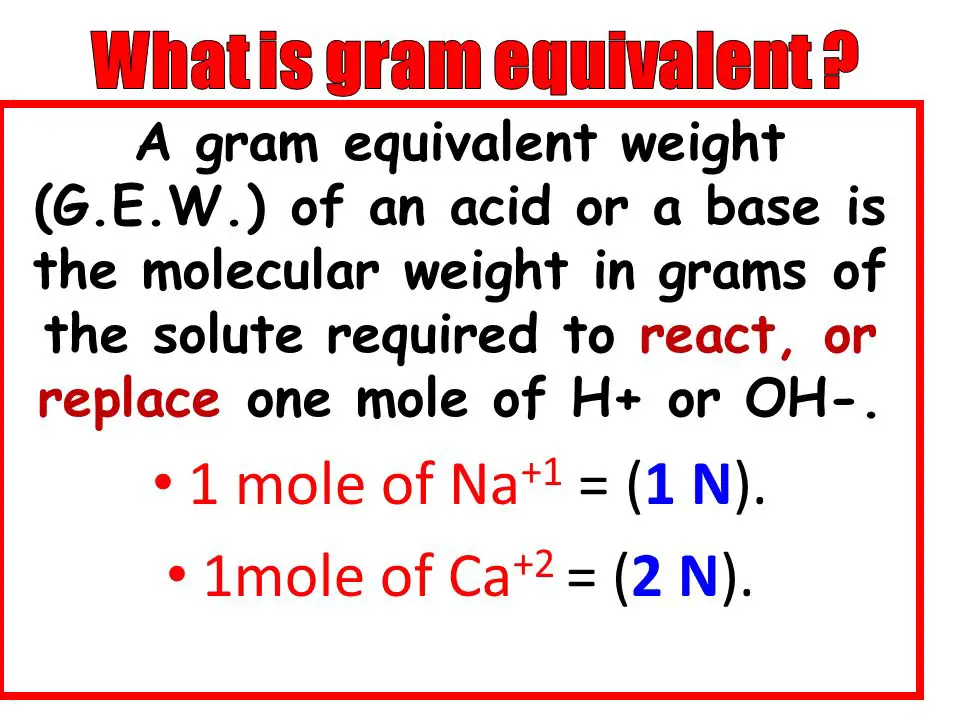
Equivalent weight is a fundamental concept in chemistry that refers to the mass of a compound that combines with or displaces a fixed amount (specifically 1.008 grams) of hydrogen. Alternatively, it is the mass that reacts with 8.0 grams of oxygen or an equivalent amount of another reactant. This measurement is crucial in the realm of stoichiometry, the field of chemistry that involves calculating the proportions of elements in chemical reactions.
Calculation Methods
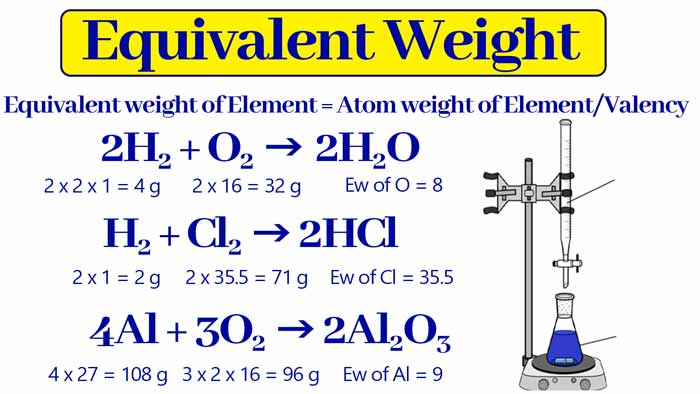
Calculating equivalent weight depends on the nature of the substance and the type of reaction it undergoes. Here’s how you can determine the equivalent weight for different scenarios:
- Acids: The equivalent weight is calculated by dividing the molar mass by the number of H+ ions the acid can donate. For instance, hydrochloric acid (HCl) donates one hydrogen ion.
- Bases: For bases, it is the molar mass divided by the number of OH- ions the base can accept. Sodium hydroxide (NaOH), which accepts one hydroxide ion, serves as a typical example.
- Salts: In salts, the equivalent weight is the molar mass divided by the number of electrons transferred per molecule in a reaction.
- Oxidizing and Reducing Agents: Here, the calculation involves the molar mass divided by the change in oxidation number per molecule in a reaction.
Example Calculations
To further elucidate the calculation of equivalent weight, consider sulfuric acid (H₂SO₄), which can donate two hydrogen ions. Its molar mass is approximately 98 g/mol. Therefore, its equivalent weight is calculated as follows:
- Equivalent Weight = Molar Mass / Number of H+ ions = 98 g/mol / 2 = 49 g/mol
This method simplifies understanding how much of a substance is needed to react with a specific amount of another chemical.
Understanding Gram Equivalent
Definition of Gram Equivalent
The gram equivalent is a measure of the reactive capacity of a molecule expressed in grams. It is a derivative concept from the equivalent weight and helps in quantifying the amount of a substance that reacts with or replaces another substance in a chemical reaction.
Formula and Application
The formula to calculate the gram equivalent is:
- Gram Equivalent = Mass of Substance (g) / Equivalent Weight (g)
This calculation is pivotal in reactions involving elements that combine in ratios other than simple whole numbers, especially in complex synthesis and analysis processes.
Practical Examples
To illustrate, if you need to determine how many grams of sodium hydroxide (NaOH) are required to neutralize 50 grams of sulfuric acid (H₂SO₄), you would use the following steps:
- Calculate the equivalent weight of H₂SO₄ (as previously calculated, 49 g/mol).
- Since NaOH has an equivalent weight of 40 g/mol (molar mass of 40 g/mol divided by 1 OH- ion), calculate the gram equivalents for each:
- H₂SO₄: 50 g / 49 g/mol = 1.02 equivalents
- NaOH required = 1.02 equivalents * 40 g/mol = 40.8 g
This calculation shows that 40.8 grams of NaOH are needed to neutralize 50 grams of sulfuric acid.
Comparison of Concepts
Key Differences Outlined
While both terms are used to describe quantities in chemical reactions, equivalent weight refers to the mass that participates in chemical reactions per equivalent of charge or atoms, whereas gram equivalent refers to the actual mass of the substance in grams based on its chemical equivalency.
When to Use Each Measurement
- Equivalent Weight: Used when discussing the theory behind reaction equations and during the educational breakdown of chemical processes.
- Gram Equivalent: Applied in practical laboratory settings and when precise measurements are required for reacting quantities.
Table of Differences
| Feature | Equivalent Weight | Gram Equivalent |
|---|---|---|
| Definition | Mass reacting with one unit of another substance | Mass in grams of a substance’s reactive capacity |
| Calculation Base | Molar mass and valency | Mass and equivalent weight |
| Usage Scenario | Theoretical discussions and calculations | Practical laboratory work and titrations |
This table helps in visually distinguishing between the two concepts, facilitating a better understanding of when and how to use each in various chemical contexts.
Applications in Chemistry
Role in Stoichiometry
Stoichiometry, the aspect of chemistry that involves the calculation of reactants and products in chemical reactions, relies heavily on concepts like equivalent weight and gram equivalent. These measurements ensure that chemical equations are balanced and that the quantities used in reactions are precise. For example, in a simple acid-base neutralization reaction, knowing the equivalent weights of the acid and the base allows chemists to calculate exactly how much of each substance is needed to achieve neutralization without any excess.
Importance in Titration
Titration, a common method used in chemistry to determine the concentration of a known reactant, also depends on these concepts. During titration:
- The equivalent weight of the titrant (the substance with a known concentration) is crucial for determining how much should be added to the analyte (the substance whose concentration is unknown) to reach the endpoint of the reaction.
- The gram equivalent helps in ensuring that the volume of titrant added is stoichiometrically equivalent to the substance being titrated, thus allowing for precise measurements of chemical concentrations.
Other Practical Uses
Beyond stoichiometry and titration, equivalent weight and gram equivalent play significant roles in:
- Manufacturing: Ensuring correct proportions in the synthesis of chemical products.
- Pharmaceuticals: Accurate dosing in drug formulation and production.
- Environmental Science: Calculating pollutants and their neutralization in environmental remediation.
Common Confusions
Equivalent Weight vs Molar Mass
A frequent confusion in chemistry involves distinguishing between equivalent weight and molar mass:
- Molar Mass is the mass of one mole of a substance (expressed in g/mol), representing the aggregate mass of its constituent atoms.
- Equivalent Weight is specifically tailored to reactions and varies according to the substance’s role in the reaction and the valence change involved.
This distinction is crucial when preparing solutions or conducting reactions where the interchange of ions or molecules depends on their equivalent rather than total mass.
Misapplications in Experiments
Misunderstanding the correct use of equivalent weight and gram equivalent can lead to significant errors in experiments. Common issues include:
- Using molar mass in place of equivalent weight in titration calculations, leading to incorrect stoichiometric relationships and unbalanced chemical reactions.
- Misinterpreting equivalent weight in redox reactions, which can result in inadequate or excess reagents being used, thereby affecting the yield and safety of the reaction.
FAQ on Chemistry Concepts
What is equivalent weight?
Equivalent weight is a concept in chemistry that refers to the mass of a substance that can either combine with or displace one equivalent of hydrogen (or another reference element). It varies depending on the type of reaction the substance undergoes and is a pivotal part of understanding chemical stoichiometry.
How do you calculate gram equivalent?
To calculate the gram equivalent, divide the mass of the substance by its equivalent weight. The resulting figure represents the reactive capacity of the substance in a chemical reaction, providing a basis for measuring reactants in a balanced chemical equation.
When should I use equivalent weight?
Equivalent weight should be used when dealing with reactions where ions or molecules combine in simple integer ratios, often observed in precipitation and redox reactions. It is particularly useful in titrations and other quantitative chemical analyses.
What is the difference between molar mass and equivalent weight?
Molar mass is the mass of one mole of a substance, whereas equivalent weight depends on the valence factor of the substance in a specific chemical reaction. The equivalent weight might be the same as the molar mass or a fraction of it, depending on the substance’s role in the reaction.
Conclusion
In conclusion, the concepts of gram equivalent and equivalent weight are central to the practice of chemistry. They provide essential metrics that help chemists and students alike understand and predict the outcomes of chemical reactions. Recognizing the differences between these measures not only aids in academic settings but also enhances the precision needed in industrial chemical applications.
Understanding and utilizing these measurements correctly can significantly impact the efficiency and accuracy of chemical experiments. As such, they are fundamental tools in the chemist’s toolkit, vital for both educational purposes and practical applications in the field.

