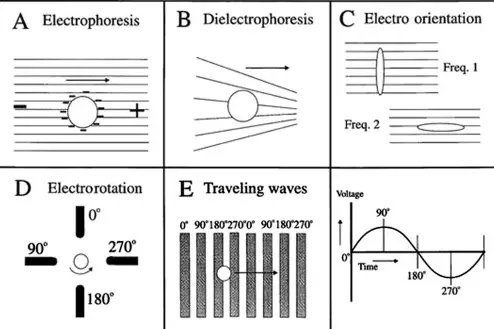
Electrophoresis and dielectrophoresis are two prominent techniques used for the separation and manipulation of particles based on their electrical properties. Both methods employ electric fields, yet they operate under different principles and are utilized in varying contexts within scientific research and industrial applications. The distinction between these two processes is crucial for professionals working in fields from biotechnology to material science.
Electrophoresis is a process that separates particles based on their charge and size by moving them through a gel or fluid under the influence of an electric field. Dielectrophoresis, on the other hand, manipulates particles by polarization effects in non-uniform electric fields, independent of their intrinsic charge. These differences influence the selection of techniques in practical applications, affecting efficiency, sensitivity, and suitability for different types of samples.
While both techniques are fundamental in the analysis and separation of molecules, their unique mechanisms and applications highlight the diverse approaches in handling particles. Understanding these can aid in choosing the appropriate method for specific scientific or industrial tasks, enhancing the efficacy of the outcomes.
Basics of Electrophoresis
What is Electrophoresis?
Electrophoresis is a laboratory technique used to separate DNA, RNA, or protein molecules based on their size and electrical charge. An electric field is applied to a gel matrix, and charged molecules migrate towards the opposite charge at rates inversely proportional to their size.
Key Components and Setup
The primary components required for electrophoresis include:
- Power supply: Delivers a steady electric current.
- Electrophoresis chamber: Holds the gel and buffer solution.
- Gel matrix: Typically made from agarose or polyacrylamide, providing a medium through which molecules can move.
- Buffer solution: Conducts the electric current and maintains a stable pH environment.
Setting up electrophoresis involves these steps:
- Assemble the gel in the chamber.
- Pour the buffer solution to cover the gel.
- Load samples into the wells of the gel.
- Connect the chamber to the power supply and run the electrophoresis.
Types of Electrophoresis
There are several types of electrophoresis, each suited to different applications:
- Agarose gel electrophoresis: Used for DNA and RNA separation.
- SDS-PAGE: Standard for separating proteins by size.
- Capillary electrophoresis: Provides high-resolution separation and is used in forensic and genetic testing.
Basics of Dielectrophoresis
Definition of Dielectrophoresis
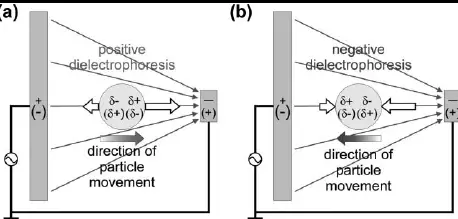
Dielectrophoresis (DEP) is a process that separates or manipulates particles suspended in a non-uniform electric field. It is unique in that it does not require the particles to be inherently charged.
Core Components and Configuration
Key components for dielectrophoresis include:
- Electrodes: Create the non-uniform electric field.
- Medium: Usually a liquid where particles are suspended.
- Power supply: Generates the electric field.
Configuration steps include:
- Design and setup of the electrode array.
- Calibration of the electric field strength.
- Suspension of particles in the medium.
Variants of Dielectrophoresis
Different DEP techniques cater to specific research needs:
- Continuous DEP: Used for continuous separation of particles.
- Traveling-wave DEP: Employs a moving electric field to transport particles.
- Insulative DEP: Uses insulating structures to shape the electric field.
Comparison Factors
Mechanism of Action
- Electrophoresis: Molecules move based on charge and size under a uniform electric field.
- Dielectrophoresis: Particles move due to polarization effects in a non-uniform electric field.
Materials and Equipment
While both techniques use similar basic equipment like power supplies and buffers, electrophoresis requires gels and dielectrophoresis uses complex electrode arrays.
Application Fields
- Electrophoresis: Widely used in molecular biology for DNA sequencing, RNA transcription analysis, and protein sizing.
- Dielectrophoresis: Common in cellular research, microfluidics, and the manipulation of nanoparticles and biological cells.
Mechanistic Differences
Physical Principles Involved
- Electrophoresis: Relies on the electrostatic force acting on charged particles.
- Dielectrophoresis: Utilizes the polarization force in non-uniform electric fields.
Movement of Particles
- Electrophoresis: Particles move in a straightforward path from negative to positive poles.
- Dielectrophoresis: Particles can be directed in complex paths due to variable field geometries.
Influence of Electric Field
- Electrophoresis: Uniform fields push particles based on their charge.
- Dielectrophoresis: Non-uniform fields create zones of high and low force, capturing or repelling particles based on their dielectric properties.
Equipment and Setup
Electrophoresis Equipment
Electrophoresis equipment is essential for conducting various types of gel electrophoresis, such as agarose and polyacrylamide gel electrophoresis. The standard setup includes:
- Power Supply: This component regulates the voltage and current that pass through the gel, dictating the speed and efficiency of the separation process.
- Gel Tanks: These containers hold the gel and buffer solution. They are designed to provide a stable environment for the electrophoresis to occur.
- Loading Combs: These tools create wells in the gel where samples are loaded. They are crucial for ensuring that samples are evenly spaced and inserted.
- Gel Documentation Systems: Post-separation, these systems capture images of the gels to analyze the results, often using UV light to visualize fluorescent markers.
Dielectrophoresis Devices
Dielectrophoresis devices are more specialized compared to traditional electrophoresis setups. Key components include:
- Microelectrode Arrays: These are sophisticated arrays that generate non-uniform electric fields required for manipulating particles.
- Control Systems: To manage the complex fields and ensure precise manipulation, advanced control systems are utilized.
- Observation Chambers: These are critical for monitoring the behavior and movement of particles within the device.
Setup Comparison
When comparing the setups for electrophoresis and dielectrophoresis, several differences become apparent:
- Complexity: Dielectrophoresis setups are generally more complex due to the need for precise control over non-uniform electric fields.
- Size and Scale: Electrophoresis equipment can handle larger sample sizes, whereas dielectrophoresis devices are often used on a much smaller scale, such as cellular or molecular levels.
- Cost: Due to the complexity and precision required, dielectrophoresis devices can be more expensive than standard electrophoresis setups.
Applications in Science
Biological Applications
In biology, electrophoresis is widely used for DNA and RNA analysis, helping in genetic mapping, forensic examinations, and cloning. Dielectrophoresis finds its application in cell sorting, especially in cancer research and stem cell studies where specific cell populations are isolated without markers.
Industrial Applications
Electrophoresis is utilized in the pharmaceutical industry for drug purity testing and protein analysis. Dielectrophoresis is applied in nanoparticle manufacturing and in the development of integrated circuits where precise particle placement is necessary.
Research and Development
Both techniques are pillars in R&D, with electrophoresis contributing to developments in genetics and molecular biology, and dielectrophoresis in emerging fields like lab-on-a-chip technologies and microfluidics.
Advantages and Limitations
Benefits of Electrophoresis
Electrophoresis offers simplicity and effectiveness. It is suitable for a wide range of biological molecules and provides high-resolution results, which are easy to interpret and reproduce.
Advantages of Dielectrophoresis
Dielectrophoresis allows for the manipulation of particles without the need for chemical labeling or surface modification, preserving the native state of the particles. It is extremely useful for the study of live cells and delicate biomolecules.
Limitations and Challenges
Despite its advantages, electrophoresis can be limited by the size of molecules it can separate, and it often requires large sample volumes. Dielectrophoresis, while precise, requires expert knowledge to design and execute experiments, limiting its accessibility.
Future Trends
Innovations in Electrophoresis
Future advancements in electrophoresis are expected to focus on increasing throughput and automation, reducing the time and labor required for analyses. Innovations might include the integration of AI to predict and control separation parameters for optimal results.
Advances in Dielectrophoresis
Advancements in microfabrication and nano-engineering are likely to enhance the capabilities of dielectrophoresis, making it more accessible and applicable in a wider range of scientific inquiries.
Predicted Developments
Both fields are poised for significant technological breakthroughs. For electrophoresis, this might involve novel gel materials and patterns that improve resolution and speed. For dielectrophoresis, newer and more efficient electrode designs could expand its use in more mainstream applications, including medical diagnostics and environmental monitoring.
Frequently Asked Questions
How does electrophoresis work?
Electrophoresis works by applying an electric field across a gel or liquid medium, causing charged particles to migrate at different speeds depending on their size and charge. This results in the separation of the particles along the medium.
What is dielectrophoresis used for?
Dielectrophoresis is primarily used for the manipulation and characterization of particles, including cells and nanoparticles. It can sort, trap, or pattern particles based on their dielectric properties without requiring them to be charged.
Can electrophoresis separate neutral molecules?
Yes, electrophoresis can separate neutral molecules if they are first treated with chemicals that confer a charge to them. This modification allows for the separation based on size and charge, even if the original molecule lacks a charge.
Is dielectrophoresis better than electrophoresis?
The choice between dielectrophoresis and electrophoresis depends on the specific application. Dielectrophoresis offers advantages in manipulating non-charged particles and can be more selective in certain contexts, whereas electrophoresis is typically faster for separating charged biomolecules like DNA or proteins.
Conclusion
Electrophoresis and dielectrophoresis serve as pivotal techniques in the separation and analysis of particles. Each method has its strengths and limitations, making them indispensable tools in scientific research and industry. Understanding their differences and applications not only enhances practical skills but also broadens the potential for innovation in various fields.
Choosing between electrophoresis and dielectrophoresis involves considering the nature of the sample and the desired outcome of the separation or manipulation. By aligning the method with the specific requirements of a task, researchers and technicians can achieve more precise and efficient results, driving forward advancements in technology and science.