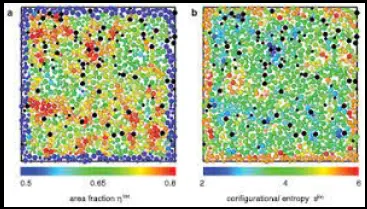
Entropy is a fundamental concept in physics that describes the degree of disorder or randomness in a system. It is a measure that helps scientists understand how systems evolve over time, particularly in terms of energy distribution and matter organization. By distinguishing between configurational and thermal entropy, researchers can delve deeper into the microscopic behavior of materials and the macroscopic manifestations of heat.
Configurational entropy refers to the randomness associated with the arrangement of particles within a system, independent of the heat energy it possesses. It is especially relevant in solid-state physics and information theory, where the arrangement of elements within a structure dictates overall system behavior. On the other hand, thermal entropy is directly related to the temperature of a system and quantifies the dispersal of energy as heat within that system, affecting its overall thermal state.
Both types of entropy are critical for understanding how systems transition between different states of energy and order. For example, configurational entropy can influence material properties like phase transitions and alloy stability, while thermal entropy is crucial for processes like heat engines and refrigeration cycles.
Entropy Basics
Defining Entropy
Entropy is a concept from thermodynamics, a branch of physics that deals with heat and temperature and their relation to energy and work. It is commonly understood as a measure of disorder or randomness in a system. The more ways particles can be arranged without affecting the system’s overall energy, the higher the entropy. This definition lays the foundation for understanding more complex behaviors in physics and engineering.
Key Concepts and Principles
Several key principles underpin the concept of entropy:
- Second Law of Thermodynamics: This law states that the entropy of an isolated system will tend to increase over time, reaching a maximum value at equilibrium.
- Statistical Mechanics: Entropy can also be viewed from a statistical perspective, where it represents the number of microscopic configurations that correspond to a macroscopic state.
- Information Theory: In this context, entropy is a measure of uncertainty or information content, often used in the encoding and transmission of data signals.
Configurational Entropy
Definition and Scope
Configurational entropy is specifically concerned with the arrangement of particles in a system and how this arrangement contributes to the system’s overall entropy. It does not consider the kinetic energy of particles (related to temperature) but focuses on the number of ways particles can be positioned.
Role in System States
In materials science, configurational entropy plays a critical role in determining the physical properties of materials. It affects:
- Phase Transitions: As the configurational entropy changes, it can induce phase transitions in materials, such as from solid to liquid or liquid to gas.
- Alloy Stability: The configurational entropy of alloys can influence their stability and the formation of different phases within the alloy.
Examples in Materials Science
- High Entropy Alloys: These are alloys designed to maximize configurational entropy to enhance material properties like strength and temperature resistance.
- Glass Formation: The configurational entropy is crucial in understanding why some materials form glasses instead of crystals when cooled.
Thermal Entropy
Definition and Context
Thermal entropy relates to the heat energy within a system and how this energy is dispersed among the system’s particles. It increases as the temperature increases, providing a measure of energy dispersal at a given temperature.
Role in Heat Transfer
Thermal entropy is fundamental in heat transfer processes, which are vital in various engineering applications:
- Engines: In thermodynamics, understanding entropy helps in designing more efficient engines and refrigerators by analyzing the energy flow and transformations.
- Climate Control: In buildings, entropy calculations can improve systems for heating and cooling, making them more energy-efficient.
Applications in Thermodynamics
Thermal entropy applications extend to:
- Power Generation: Thermal entropy analysis helps optimize the operation of power plants, focusing on converting heat into electrical energy efficiently.
- Waste Heat Recovery: Systems designed to reclaim waste heat and convert it to useful energy rely on principles of thermal entropy to maximize recovery.
Comparative Analysis
Configurational vs. Thermal
While both types of entropy relate to disorder and energy dispersion, configurational entropy deals with particle arrangement without considering energy changes, whereas thermal entropy focuses on heat energy distribution within a system.
Impact on System Behavior
- Material Properties: Configurational entropy affects material structure and phase behavior, while thermal entropy influences material reactions to heat.
- System Efficiency: In thermodynamic cycles, reducing thermal entropy increases efficiency, whereas configurational entropy can help optimize material properties for specific applications.
Measurement Techniques
Measuring both types of entropy involves different approaches:
- Calorimetry: Used primarily for thermal entropy, measuring the heat change and temperature to calculate entropy change.
- X-ray Diffraction: Useful for configurational entropy, as it helps determine particle arrangements in solid materials.

Practical Implications
Engineering Applications
Entropy concepts are crucial in engineering, particularly in designing systems that are energy-efficient and sustainable. The application of entropy principles in engineering includes:
- Thermal Systems: Engineers use entropy calculations to optimize boilers, heat exchangers, and refrigeration units, aiming for maximum energy transfer with minimal energy loss.
- Material Design: Understanding both configurational and thermal entropy helps in developing new materials with desired mechanical and thermal properties, such as lightweight composites for aerospace applications.
Computational Modeling
Computational modeling is another area where entropy plays a significant role, enabling the simulation of complex systems to predict behavior under various scenarios:
- Molecular Dynamics: Models that simulate the behavior of atoms and molecules often incorporate entropy calculations to predict material properties at different temperatures and pressures.
- Climate Modeling: Entropy is used in climate models to help predict how energy imbalances affect earth systems, crucial for understanding global warming and weather patterns.
Theoretical Perspectives
Statistical Mechanics Insights
Statistical mechanics provides a framework for understanding entropy beyond the macroscopic descriptions of classical thermodynamics:
- Ensemble Theory: This aspect of statistical mechanics uses the concept of an ensemble, a collection of microstates, to calculate the entropy of a system statistically.
- Entropy and Information: The link between entropy and information theory is explored through statistical mechanics, demonstrating how entropy measures the uncertainty or the spread of possible microstates.
Quantum Mechanics Contributions
Quantum mechanics has contributed significantly to our understanding of entropy, especially at atomic and subatomic levels:
- Quantum Entanglement and Entropy: Studies show that entropy can quantify the degree of entanglement in a quantum system, with higher entropy indicating more complex entanglements.
- Quantum Computing: In quantum computing, entropy measures are used to analyze the “noise” within quantum systems, affecting computation accuracy and stability.
Future Directions
Research Trends
Current research trends in entropy include:
- Non-equilibrium Systems: Advanced studies focus on entropy generation in systems away from equilibrium, which is essential for understanding biological processes and innovative materials.
- Entropy in Biology: Researchers are exploring entropy in biological systems, examining how entropy production helps maintain life processes, from cellular function to ecological systems.
Technological Innovations
Technological advancements driven by entropy research include:
- Advanced Thermal Management: New materials and methods for managing heat in electronics and photonics are being developed using insights from entropy studies.
- Energy Recovery Systems: Technologies that capture and reuse waste heat from industrial processes are improving, thanks to better understanding of entropy dynamics.
Frequently Asked Questions
What is Configurational Entropy?
Configurational entropy measures the number of ways particles can be arranged in a system, maintaining the same energy level. It highlights the microscopic configurations that might not affect the system’s overall energy but alter its structural properties.
How is Thermal Entropy Measured?
Thermal entropy is measured by the amount of heat energy distributed per unit temperature in a system. This measurement helps in understanding energy efficiency and the entropy changes during heat transfer processes.
Why are Both Entropies Important?
Both configurational and thermal entropy are essential for a comprehensive understanding of how energy and matter interact in different physical contexts. They help predict the behavior of systems under various thermal and structural conditions.
Can Entropy Be Reversed?
In theory, entropy is a measure that tends to increase over time, according to the second law of thermodynamics. However, local decreases in entropy are possible under specific conditions, though these do not violate the overall trend towards greater disorder.
Conclusion
In summary, configurational and thermal entropy are pivotal concepts that help elucidate the complex behaviors of systems under various conditions. Their distinction is not merely academic but plays a crucial role in practical applications across multiple scientific and engineering disciplines. Understanding these forms of entropy enables better prediction and manipulation of material and thermal properties.
As the scientific community continues to explore the nuances of entropy, the distinction between its configurational and thermal forms will remain a key area of research. These insights not only enhance our theoretical knowledge but also improve the technological applications that drive innovation in materials science and thermodynamic systems.