Calcium ammonium nitrate and ammonium nitrate are two compounds used as fertilizers in agriculture, but they have many differences. In this blog post, we will explore the main differences between calcium ammonium nitrate and ammonium nitrate, including their composition, uses, and benefits. We will also discuss how these compounds can be used to improve the yield and quality of crops.
We will also discuss how these compounds can be used to improve the yield and quality of crops.
Overview of calcium ammonium nitrate
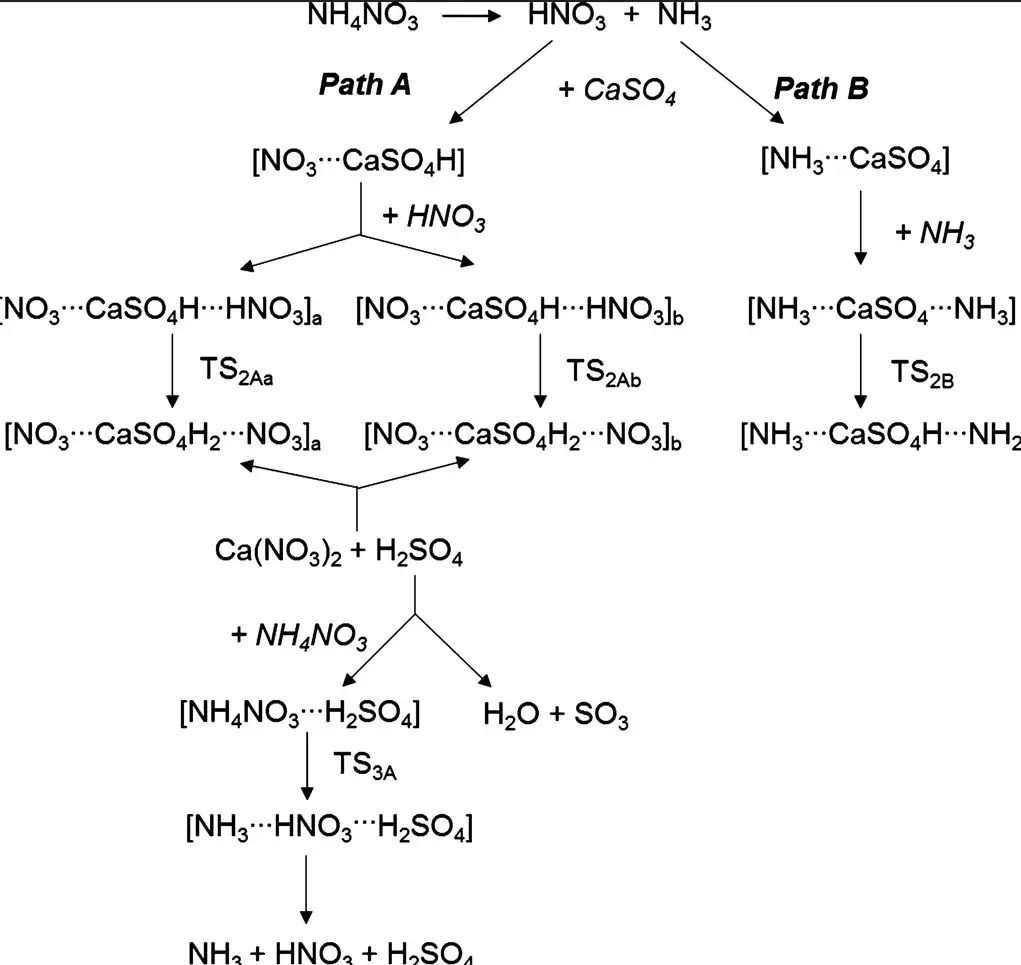
Calcium ammonium nitrate (CAN) is a fertilizer composed of calcium, nitrogen, and oxygen, which is mainly used to supply nitrogen to crops. It is a compound of ammonium nitrate (NH4NO3) and calcium carbonate (CaCO3) and is often referred to as “nitro-limestone” or “cal-ammonium nitrate. ” While it appears similar to ammonium nitrate on the surface, there are several key differences.
” While it appears similar to ammonium nitrate on the surface, there are several key differences. CAN is more water-soluble than ammonium nitrate, meaning it can be more easily absorbed by plants. Additionally, CAN has a higher nitrogen content, making it a better choice for crops in need of a nitrogen boost.
CAN also contains calcium, providing a valuable nutrient for plants. Finally, CAN has a lower acidity than ammonium nitrate, making it safer to use in soils that are already acidic.
Overview of ammonium nitrate
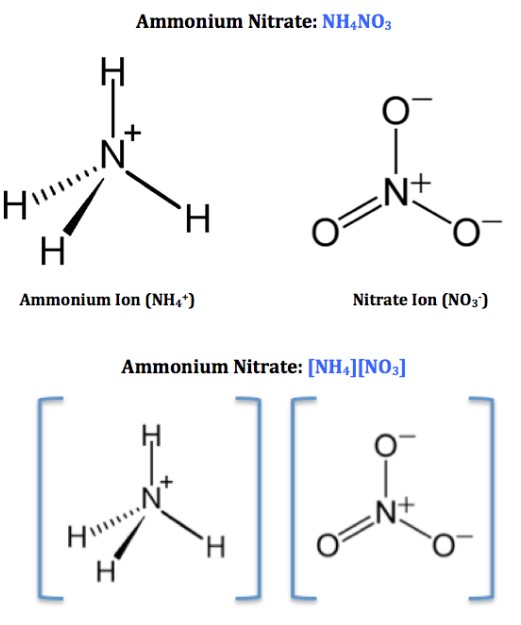
Ammonium nitrate is a chemical compound commonly used in agriculture as a fertilizer. It is made up of two elements, nitrogen and hydrogen, and is used to provide nitrogen to soil and plants.
When combined with calcium, it forms a product known as calcium ammonium nitrate (CAN). The difference between CAN and ammonium nitrate is in the way they are used. CAN is used as a fertilizer to supply nitrogen to plants, while ammonium nitrate is used as a fertilizer to supply nitrogen to soil.
This difference is due to the fact that CAN also contains calcium, which helps to balance the plant’s pH levels and prevent nitrogen burn. Additionally, CAN has a slower release rate, meaning it can provide nitrogen to the soil and plants over a longer period of time.
Key differences between calcium ammonium nitrate and ammonium nitrate
Calcium ammonium nitrate (CAN) and ammonium nitrate (AN) are two different nitrogen fertilizers commonly used for agricultural purposes. Both of these fertilizers contain nitrogen, which is essential to the growth of plants, but there are some key differences between them.
CAN is also slightly more acidic than AN, and is therefore better suited for soils with a higher pH level. CAN is also more soluble than AN, meaning it dissolves more quickly in water, and is therefore a better choice for farmers who need to apply fertilizer quickly.
Finally, CAN is more expensive than AN, so it may not be the best choice for farmers with limited budgets.
Applications of calcium ammonium nitrate and ammonium nitrate
Calcium ammonium nitrate and ammonium nitrate are both nitrogen-based fertilizers, but they have some key differences. Calcium ammonium nitrate is a blend of two fertilizers, calcium nitrate and ammonium nitrate.
Ammonium nitrate, on the other hand, is a single fertilizer that is higher in nitrogen but lacks the calcium component. Applications of calcium ammonium nitrate include improving the quality of the soil, increasing nitrogen availability for plant growth, and providing other essential nutrients.
Ammonium nitrate is often used to increase the nitrogen content of soil and is also found in explosives. It’s important to note that while both fertilizers have their uses, they should be used sparingly, as too much can cause soil damage.
Advantages and disadvantages of calcium ammonium nitrate and ammonium nitrate
The two most common forms of nitrogen fertilizer are calcium ammonium nitrate (CAN) and ammonium nitrate (AN). Both of these fertilizers are essential for providing the nitrogen that plants need to grow, but they each have their own advantages and disadvantages. CAN is a slow-release fertilizer, meaning that the nutrients are slowly released into the soil over time, helping plants to receive a steady stream of nutrition.
This helps reduce the risk of over-fertilizing and burning plants. Furthermore, CAN has a high content of calcium, which is beneficial for soils and plants with a calcium deficiency.
On the other hand, CAN is more expensive than AN, and it is not suitable for acid-loving plants. AN is a more concentrated form of nitrogen and is released into the soil more quickly than CAN.
However, it can be more difficult to control the amount of nitrogen released, making it more likely to burn plants. Additionally, AN does not contain any calcium, so it is not suitable for calcium-deficient soils.
In conclusion, both CAN and AN are effective forms of nitrogen fertilizer, and each has its own advantages and disadvantages. Choosing the right fertilizer for your plants depends on the type of plants you are growing, the soil conditions, and the desired release rate.
Final Touch
Calcium ammonium nitrate and ammonium nitrate are both nitrogen-based fertilizers, but they have significant differences. Calcium ammonium nitrate contains calcium as well as nitrogen, which helps to reduce soil acidity, while ammonium nitrate does not.
Calcium ammonium nitrate also releases nitrogen more slowly than ammonium nitrate, providing a more consistent nutrient supply for plants. In terms of cost, calcium ammonium nitrate is more expensive than ammonium nitrate. Both fertilizers must be used with caution, as nitrogen can be toxic to plants and the environment.
