Borazine and diborane are two significant compounds in the realm of inorganic chemistry, each bearing unique characteristics and applications. While they share a connection through the element boron, their properties, uses, and behaviors differ markedly. This contrast not only piques the interest of chemists but also has practical implications in various scientific and industrial fields.
Borazine, often referred to as “inorganic benzene,” mimics the ring structure of benzene but consists of alternating boron and nitrogen atoms. Diborane, on the other hand, is a highly reactive gas composed of boron and hydrogen atoms, known for its role in organic synthesis reactions. These distinctions highlight the versatility of boron-containing compounds and their potential for wide-ranging applications from materials science to pharmaceutical synthesis.
Exploring the differences between borazine and diborane reveals their structural variances and contrasting chemical behaviors. Such insights not only contribute to academic knowledge but also guide practical applications, influencing how these compounds are synthesized, manipulated, and utilized across various scientific disciplines.
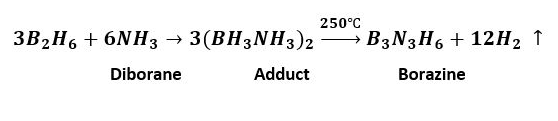
Borazine Basics
Chemical Composition
Borazine, with the molecular formula B₃H₆N₃, is an inorganic compound comprised of boron, nitrogen, and hydrogen atoms. This structure forms a hexagonal ring, resembling that of benzene, but substitutes every alternate carbon atom with a boron or a nitrogen atom. The boron and nitrogen atoms in borazine are linked alternately by single bonds, which is pivotal in imparting borazine with unique properties not typically seen in organic compounds.
Physical Properties
Borazine is distinguished by its stability and resistance to heat, characteristics that are exceptionally valuable in high-performance materials. It is a colorless liquid at room temperature, with a boiling point of about 53 degrees Celsius, which is relatively low compared to many similar organic compounds. This low boiling point is due to the polarity of the boron-nitrogen bonds, which are less electron-dense compared to carbon-carbon bonds in benzene. Moreover, borazine is insoluble in water but soluble in nonpolar solvents, which further showcases its similarity to organic solvents like benzene.
Synthesis Methods
The synthesis of borazine typically involves the reaction of diborane (B₂H₆) with ammonia (NH₃), a process that occurs under controlled conditions:
- Mix diborane and ammonia gases in a 1:1 molar ratio.
- Heat the mixture at a temperature of approximately 250 degrees Celsius.
- The reaction is exothermic, leading to the formation of borazine and hydrogen gas as by-products.
This method highlights the simplicity yet efficiency in producing borazine, albeit with careful control of reaction conditions to ensure safety and purity of the product.
Diborane Basics
Chemical Composition
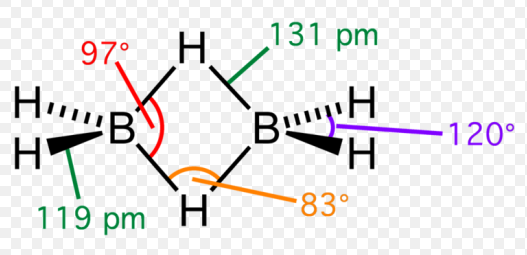
Diborane, or B₂H₆, consists of two boron atoms bonded together with six hydrogen atoms. What makes diborane stand out is its structure; it contains four terminal hydrogen atoms and two bridging hydrogen atoms, creating a unique “banana bond” that is central to its reactivity. This bonding arrangement is not only rare but also illustrates the complexity of boron chemistry.
Physical Properties
Diborane is a gas at room temperature and is noted for its extreme reactivity and flammability. It has a boiling point of -92.5 degrees Celsius, which underscores its volatility. Diborane is colorless and has a distinct sweet odor, though it is dangerously toxic if inhaled. Its lightness and reactivity make it a challenging compound to work with, requiring specific storage and handling techniques to prevent its decomposition or accidental ignition.
Synthesis Methods
The synthesis of diborane is commonly achieved through several routes, the most prominent being:
- Hydroboration: Reaction of alkynes or alkenes with borane (BH₃) in the presence of hydrogen gas.
- Reduction of boron halides: For example, boron trifluoride (BF₃) reacted with lithium hydride (LiH).
These methods highlight the importance of careful control and knowledge of reactive boron chemistry to safely produce and use diborane.

Comparative Analysis
Structural Differences
Comparing borazine and diborane, the most striking difference lies in their molecular structures. Borazine forms a stable ring similar to benzene, thus allowing it to mimic the properties of an aromatic compound. Diborane, however, features a more open and reactive structure due to its bridging hydrogen atoms. This structural variance fundamentally affects their respective chemical properties and uses.
Chemical Behavior
Borazine’s stability allows it to participate in reactions that typically involve aromatic compounds, such as electrophilic substitution, without decomposing. Diborane, conversely, is much more reactive, engaging in complex reactions such as hydroboration, where it adds across double bonds in organic molecules, a key step in synthetic organic chemistry.
Reactivity with Other Compounds
The reactivity of diborane with oxygen or moisture can lead to explosive results, highlighting its sensitivity and the need for stringent handling protocols. Borazine, while more chemically stable, reacts at high temperatures to form boron nitride, a compound prized in materials science for its hardness and thermal stability. These reactions underscore the different applications and precautions necessary when working with these two boron compounds.

Applications and Uses
Borazine in Industry
Borazine’s chemical similarity to benzene allows it to serve as a versatile precursor in the synthesis of boron nitride, a material renowned for its hardness and thermal stability. This transformation is crucial in industries requiring materials that can withstand extreme temperatures and abrasive conditions. Additionally, borazine is used in the creation of:
- Advanced ceramics: These materials benefit from borazine’s stability and heat resistance.
- Semiconductor layers: Borazine-derived coatings are employed in electronics for their insulative properties.
- High-temperature lubricants: Borazine’s stability under thermal stress makes it ideal for applications involving high heat.
Diborane in Research
Diborane plays a pivotal role in organic synthesis and materials science due to its unique ability to form bonds with carbon. Its applications in research include:
- Hydroboration reactions: Diborane adds across carbon-carbon double bonds to form trialkylboranes, key intermediates in organic synthesis.
- Doping of semiconductors: Diborane is used to introduce boron into semiconductors, altering their electrical properties.
The substance’s reactivity is not only a challenge but also a boon to researchers looking to manipulate molecular structures in innovative ways.
Safety and Handling
Handling Borazine
Despite borazine’s relative stability, it requires careful handling due to its potential decomposition into toxic by-products at high temperatures. Key handling guidelines include:
- Use in well-ventilated areas: To avoid accumulation of any toxic vapors.
- Storage in inert atmosphere: Borazine should be stored under nitrogen or argon to prevent degradation.
- Personal protective equipment (PPE): Gloves and goggles should be worn when handling borazine.
Handling Diborane
Diborane’s high reactivity and flammability necessitate stringent safety measures:
- Use in fume hoods: Handling should occur in a controlled environment with proper ventilation.
- Storage under cold conditions: Diborane should be kept in refrigerated, airtight containers to prevent decomposition.
- Emergency readiness: Facilities should have protocols in place for dealing with accidental releases or fires.
Safety Precautions
Both compounds demand a high level of precaution due to their toxic and reactive natures:
- Regular training: Handlers should undergo frequent safety training.
- Leak detection systems: These should be installed where gases are stored or used.
- First aid and emergency kits: Should be readily available in areas where exposure risks exist.
Environmental Impact
Borazine Effects
While borazine itself does not pose significant environmental hazards, its decomposition products can be harmful if released into the atmosphere. It’s imperative to manage emissions and waste products carefully, especially in industrial settings where large quantities are used.
Diborane Effects
Diborane’s impact on the environment is more pronounced due to its toxicity and potential for rapid oxidation. Releases can lead to:
- Air quality degradation: Diborane can react in the atmosphere to form hazardous by-products.
- Water and soil contamination: If diborane leaks, it can contaminate local ecosystems.
Controlling diborane emissions is crucial for minimizing environmental and health risks.
Future Directions
Research Trends
The future of borazine and diborane research is geared towards enhancing their utility while minimizing associated risks. Innovations focus on:
- Safer synthesis methods: Reducing the hazards involved in producing these compounds.
- Enhanced applications: Expanding their use in electronics, nanotechnology, and renewable energy sectors.
Potential Applications
Emerging applications of borazine and diborane are anticipated in areas such as:
- Improved semiconductor devices: Leveraging their unique properties to enhance device performance and efficiency.
- Advanced composite materials: Using borazine-derived materials in aerospace and automotive industries for lighter, more durable components.
FAQs
What is borazine used for?
Borazine is utilized primarily in the creation of high-temperature polymers, ceramics, and semiconductor layers. Its ability to form stable structures similar to benzene, yet with higher resistance to heat, makes it invaluable in the manufacture of heat-resistant materials.
How is diborane typically synthesized?
Diborane is synthesized through several methods, but the most common involves the reaction of boron trifluoride (BF3) with hydrogen gas under specific conditions. This process is crucial for producing diborane in a form suitable for industrial and research applications.
Are borazine and diborane safe to handle?
Both compounds require careful handling due to their reactive natures. Borazine, while more stable than diborane, can decompose at high temperatures, releasing toxic gases. Diborane is highly flammable and toxic, necessitating stringent safety measures during storage and use.
What environmental impacts do these compounds have?
The environmental impact of borazine and diborane is primarily associated with their synthesis and disposal processes. Diborane, in particular, poses risks due to its toxicity and reactivity, potentially leading to air and water pollution if not managed correctly.
Conclusion
The exploration of borazine and diborane underscores the complexity and utility of boron-based chemistry. Each compound exhibits distinct properties that make them suitable for specific applications, from advanced materials to synthesis methodologies. Their study not only enriches our understanding of chemical science but also advances the development of innovative technologies.
The future of research in compounds like borazine and diborane is promising, driven by the continuous quest for materials that can withstand extreme conditions and contribute to sustainable practices. As chemistry evolves, the roles of such substances are likely to expand, underscoring their significance in both scientific inquiry and practical application.