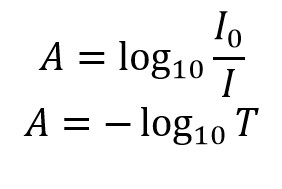When light interacts with a material, it can be absorbed, reflected, or transmitted, leading to fascinating phenomena observable in our daily lives and critical in scientific research. The concepts of absorbance and transmittance are at the heart of understanding how light behaves when it encounters different substances. These principles are not only foundational in the field of optics but also play a pivotal role in various applications ranging from environmental monitoring to medical diagnostics.
The relationship between absorbance and transmittance is straightforward yet profound. Absorbance measures how much light a material absorbs, while transmittance quantifies how much light passes through the material. Essentially, as absorbance increases, transmittance decreases and vice versa. This inverse relationship is key to analyzing and interpreting the interaction of light with materials, offering insights into the properties of the substance under investigation.
Focusing on the interplay between these two properties unveils the material’s characteristics, such as concentration, thickness, and even molecular structure. By quantifying absorbance and transmittance, scientists and engineers can deduce critical information about a substance, facilitating advancements in technology, quality control in manufacturing, and breakthroughs in scientific research.
Light Basics
Nature of Light
Light, a form of electromagnetic radiation, is both a wave and a particle. This dual nature allows it to exhibit properties such as reflection, refraction, and diffraction. Visible light, a small portion of the electromagnetic spectrum, enables us to perceive the world in color and detail.
Light Interaction with Materials
When light encounters a material, it can be absorbed, transmitted, or reflected. The outcome depends on the material’s properties and the wavelength of the light. For instance, glass transmits visible light but absorbs ultraviolet light, which is why you can see through windows but still get sunburned indoors if you’re near one on a sunny day.
Absorbance Explained
Definition and Significance
Absorbance, often denoted as �A, measures a material’s capacity to absorb light at a specific wavelength. It is a key concept in spectrophotometry, a technique used to quantify the concentration of a solute in a solution based on light absorption. The more a substance absorbs light, the higher its concentration can be inferred to be.
How Absorbance is Measured
To measure absorbance, a spectrophotometer is used. The steps involved are:
- A light source emits a beam of light at a specific wavelength.
- The light passes through a sample.
- The intensity of the transmitted light is measured.
- The absorbance is calculated by comparing the intensity of the light before and after it passes through the sample, using the formula �=−log10(�)A=−log10(T), where �T is the transmittance.
Transmittance Defined
Understanding Transmittance
Transmittance (�T) quantifies the amount of light that passes through a material. Expressed as a percentage, it reflects how transparent a material is. High transmittance means more light gets through, indicating low absorption, and vice versa.
Measurement Techniques
Transmittance is measured using a spectrophotometer, similar to absorbance. The process involves:
- Illuminating the sample with a light beam.
- Measuring the intensity of light before and after the sample.
- Calculating transmittance as the ratio of transmitted light intensity to the initial light intensity, often presented as a percentage.
Relationship Overview
Fundamental Connection
Absorbance and transmittance are inversely related. This means when the absorbance of a material increases, its transmittance decreases. This relationship is crucial in materials science, chemistry, and physics for characterizing substances.
Mathematical Relationship
The mathematical relationship between absorbance and transmittance is given by the Beer-Lambert Law, which states �=−log10(�)A=−log10(T). This formula establishes a direct correlation between the two properties, allowing for the quantification of a substance’s concentration in a solution by measuring its absorbance or transmittance.
Factors Influencing Absorbance and Transmittance
Material Properties
The chemical composition and physical structure of a material significantly affect its absorbance and transmittance. Materials with higher densities or those that are colored tend to have higher absorbance rates.
Wavelength of Light
The absorbance and transmittance of a material can vary with the wavelength of light. Some materials might be transparent to one region of the spectrum and opaque to another. This dependency is utilized in creating filters and sensors that selectively block or pass certain wavelengths.
Path Length of the Sample
The thickness of the material through which light travels, also known as the path length, directly impacts both absorbance and transmittance. Longer path lengths generally result in higher absorbance and lower transmittance, assuming the material’s composition remains constant. This principle is fundamental in designing optical devices and understanding light behavior in different media.
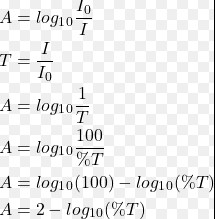
Applications
Scientific Research
In the realm of scientific exploration, understanding the interaction of light with substances is critical. Scientists employ absorbance and transmittance measurements to unlock mysteries at the molecular level. For instance, biochemists analyze DNA or protein concentrations using these principles, while material scientists rely on them to investigate the optical properties of novel materials. This not only broadens our scientific knowledge but also paves the way for groundbreaking discoveries in medicine, nanotechnology, and quantum computing.
Industrial Applications
In industry, the concepts of absorbance and transmittance find practical applications in quality control and product development. The food and beverage sector utilizes these measurements to ensure the consistency and safety of products by analyzing the concentration of various ingredients and contaminants. Similarly, the pharmaceutical industry relies on these methods to precisely quantify drug components, ensuring efficacy and compliance with stringent regulatory standards.
Environmental Monitoring
Environmental scientists use absorbance and transmittance measurements to monitor air and water quality. By detecting the presence of pollutants and toxic substances, they can assess the health of ecosystems and the safety of drinking water. This information is crucial for informing public health decisions, shaping environmental policies, and mitigating pollution.
Calculating Absorbance from Transmittance
Formula and Example Calculation
The relationship between absorbance and transmittance is governed by the Beer-Lambert Law, which can be used to calculate one from the other. The formula, �=−log10(�)A=−log10(T), where �T is the transmittance expressed as a decimal, provides a straightforward method for this calculation.
For example, if the transmittance of a solution is measured to be 40% (or 0.40 in decimal form), the absorbance can be calculated as follows:
- �=−log10(0.40)A=−log10(0.40)
- �≈0.398A≈0.398
This calculation indicates that the solution has a moderate level of absorbance, useful in determining the concentration of the solute.
Practical Considerations
Instrument Calibration
To ensure accuracy in measurements, instruments must be regularly calibrated. Calibration involves adjusting the spectrophotometer with known standards to ensure that measurements are consistent and reliable. This process helps to eliminate errors and biases that could affect the outcomes of experiments or product quality assessments.
Sample Preparation
Preparing samples correctly is fundamental to obtaining accurate absorbance and transmittance measurements. This involves:
- Ensuring the purity of the sample to prevent interference.
- Adjusting the concentration of the sample to fall within the optimal range for measurement.
- Using clean, clear cuvettes to avoid scattering or absorbing light unnecessarily.
Following these steps minimizes errors and ensures that the data collected is a true representation of the sample’s properties.
Limitations and Accuracy
While absorbance and transmittance measurements are invaluable tools, they come with limitations. Factors such as instrument sensitivity, human error in sample preparation, and inherent properties of the material being tested can influence accuracy. Recognizing these limitations is essential for interpreting results correctly and making informed decisions based on the data.
Advances and Innovations
New Technologies in Measurement
The field of optical measurement has seen significant advances, with new technologies enhancing the precision and range of applications. Innovations such as fiber-optic spectrophotometers and microfluidic devices allow for measurements in real-time and in smaller, more difficult-to-access environments. These advancements are not only expanding the possibilities for research and industrial applications but also increasing the efficiency and accuracy of measurements.
Future Directions in Research
Looking ahead, research in the field of absorbance and transmittance is moving towards integration with artificial intelligence and machine learning. These technologies have the potential to revolutionize how data is analyzed, enabling the discovery of patterns and insights that were previously unattainable. Furthermore, the development of more sensitive and selective materials for optical measurements is anticipated, which could lead to breakthroughs in environmental monitoring, healthcare diagnostics, and materials science. The future of absorbance and transmittance measurement is bright, with ongoing innovations promising to expand our understanding and capabilities in exciting new directions.
Frequently Asked Questions
What is absorbance in simple terms?
Absorbance is a measure of the amount of light that a material captures and does not allow to pass through. It indicates how much of the incident light is absorbed by the material, with higher absorbance values corresponding to greater amounts of light being absorbed. This property is crucial for determining the concentration of substances in a solution and for various analytical techniques in chemistry and biology.
How is transmittance related to absorbance?
Transmittance is directly related to absorbance through an inverse relationship. When a material has high absorbance, it means it absorbs more light, resulting in lower transmittance or less light passing through the material. This relationship is described mathematically by the Beer-Lambert Law, which is fundamental in spectrophotometry, allowing for the quantification of substance concentration by measuring light intensity before and after passing through a sample.
Why is understanding absorbance and transmittance important?
Understanding the relationship between absorbance and transmittance is essential for interpreting the interaction of light with materials. This knowledge is pivotal in fields such as analytical chemistry, environmental science, and medical diagnostics. It enables the determination of the concentration of solutions, identification of substances based on their spectral signatures, and assessment of material properties, which are critical for quality control, pollution monitoring, and disease diagnosis.
Conclusion
In sum, the intricate dance between absorbance and transmittance reveals much about the world around us, from the purity of the water we drink to the health of our bodies at the molecular level. These concepts serve as fundamental tools in the hands of scientists and engineers, enabling the detailed analysis and understanding of materials and substances across a broad spectrum of disciplines.
As we continue to explore the limits of what can be measured and understood through these optical properties, the future promises even greater insights and advancements. The study of absorbance and transmittance not only deepens our comprehension of the physical world but also propels us toward novel applications and technologies, demonstrating the enduring importance of these fundamental principles.