Acids are an essential part of chemistry and have a variety of uses. Monoprotic and diprotic acids are two different types of acids that have unique properties and are used in different ways. In this blog, we will discuss the differences between monoprotic and diprotic acids, including their structures, reactivity, and uses.
In this blog, we will discuss the differences between monoprotic and diprotic acids, including their structures, reactivity, and uses.
Definition of monoprotic and diprotic acids
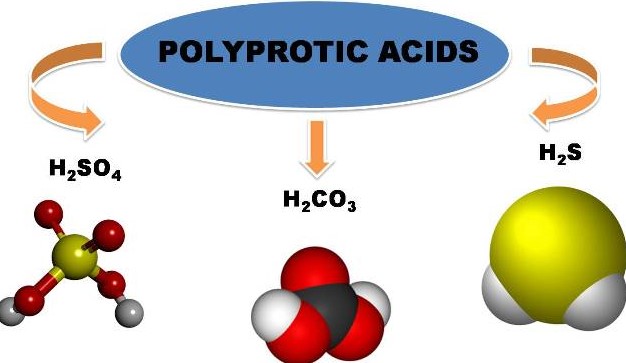
Monoprotic and diprotic acids are two types of acids that differ in how many hydrogen ions they can donate to a solution. Monoprotic acids are acids that can donate one hydrogen ion per molecule, while diprotic acids are acids that can donate two hydrogen ions per molecule. The difference between the two types is that monoprotic acids can only donate one hydrogen ion, while diprotic acids can donate two.
The difference between the two types is that monoprotic acids can only donate one hydrogen ion, while diprotic acids can donate two. This makes diprotic acids stronger because they can donate more hydrogen ions and consequently make the solution more acidic.
Properties of monoprotic and diprotic acids
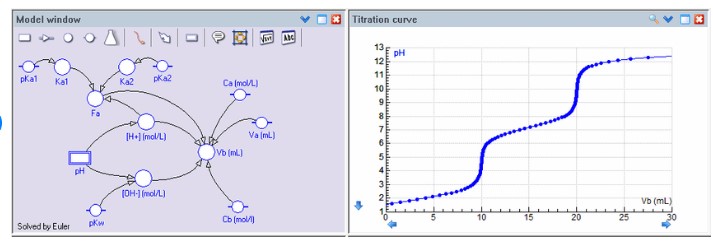
Monoprotic and diprotic acids are two types of acids that differ in their properties, with the former having one hydrogen atom that can be released and the latter having two. Monoprotic acids are weaker than diprotic acids, and typically have a pH of between 1 and
Diprotic acids, on the other hand, have a pH of between 1 and 4 and release hydrogen quickly and easily, making them more reactive and corrosive. These differences in properties make monoprotic acids ideal for use in controlling pH levels in solutions, while diprotic acids are better suited for cleaning and industrial applications.
Examples of monoprotic and diprotic acids
The key difference between monoprotic and diprotic acids is in the number of hydrogen ions they can release when dissolved in water. Monoprotic acids can release one hydrogen ion, while diprotic acids can release two hydrogen ions. Examples of monoprotic acids include hydrochloric acid, sulfuric acid, and acetic acid.
Examples of diprotic acids include carbonic acid and oxalic acid. Monoprotic acids tend to have stronger acidity than diprotic acids, as they require less energy to dissociate into ions.
However, diprotic acids can still be quite strong in certain situations and can be used to neutralize basic solutions.
Differences between monoprotic and diprotic acids
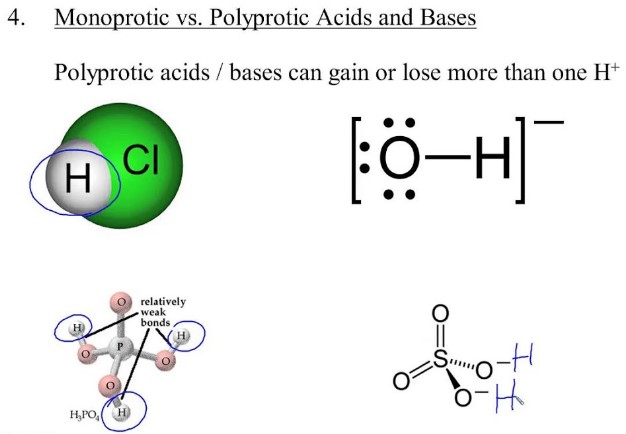
Acids are molecules that can donate hydrogen ions, or protons, when dissolved in water. Monoprotic acids are acids that donate one proton per molecule, while diprotic acids donate two protons per molecule. Understanding the differences between these two types of acids is important for a variety of applications, from industrial processes to chemical reactions.
Understanding the differences between these two types of acids is important for a variety of applications, from industrial processes to chemical reactions. Monoprotic acids are generally weaker than diprotic acids, as they have fewer protons to donate. This means that monoprotic acids require a higher concentration of the acid to reach the same level of acidity as a diprotic acid.
Additionally, monoprotic acids have a higher affinity for bases, meaning that they are more likely to react with them. Diprotic acids, on the other hand, have a lower affinity for bases and are more likely to give up their two protons to other molecules than monoprotic acids.
Understanding the differences between monoprotic and diprotic acids is essential for any chemist or researcher.
Applications of monoprotic and diprotic acids
Monoprotic and diprotic acids are two important types of acids that have a variety of applications in chemistry and industry. Monoprotic acids are acids that can donate one proton per molecule, while diprotic acids are acids that can donate two protons per molecule.
The difference between these two types of acids is the number of protons they can donate and their resulting chemical behavior. Monoprotic acids, such as hydrochloric acid, are typically more reactive than diprotic acids, such as sulfuric acid, and can be used in a variety of applications including in cleaning agents, fertilizers, and other industrial processes. Diprotic acids, on the other hand, are less reactive and are most often used in the production of fertilizers and other industrial processes that require a slower but more consistent reaction.
Final Touch
In conclusion, the main difference between monoprotic and diprotic acids is that monoprotic acids contain one acidic hydrogen atom which is released during the dissociation process, while diprotic acids contain two acidic hydrogen atoms which are released in two separate dissociation processes. Monoprotic acids are generally weaker than diprotic acids, as they require two processes to release their full potential.