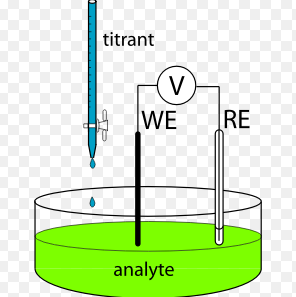
Titration stands as a pivotal technique in chemical analysis, serving as a bridge between theoretical chemistry and practical applications. This process involves measuring the concentration of an unknown solution by adding a solution of known concentration until a reaction reaches completion. Different methods of titration cater to various analytical needs, with volumetric and potentiometric titration being two prominent examples. Each method offers unique advantages and serves specific analytical purposes.
Volumetric titration is a method where the volume of a titrant required to complete a reaction is measured. Potentiometric titration, on the other hand, involves the measurement of the voltage change of the solution. These methods are fundamental in fields ranging from environmental science to pharmaceuticals, providing essential data on substance purity and concentration.
Both methods employ different techniques to reach what is known as the endpoint, the point at which the reaction is complete. Volumetric titration typically uses a color indicator to signal the endpoint, while potentiometric titration uses an electrode to detect voltage changes. Understanding these differences is crucial for choosing the appropriate titration method for a given chemical analysis.
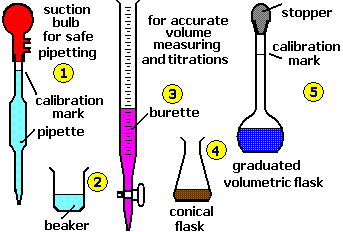
Basics of Titration
Definition and Principles
Titration is a quantitative chemical analysis method used to determine the concentration of an identified analyte. By adding a reagent solution—known as the titrant—to the analyte solution until the reaction reaches a known completion point, titration helps establish the precise measure of the unknown concentration. This point, known as the endpoint, is often marked by a significant change in the color or electrical properties of the solution.
The underlying principle of titration involves a precise and controlled addition of various solutions. During the process, the amount of titrant reacted with the analyte is measured and used to calculate the analyte’s concentration based on the stoichiometry of the chemical reaction.
Common Applications
Titration is widely employed in various sectors, including:
- Pharmaceuticals: Ensuring drug safety and dosage accuracy.
- Environmental Science: Measuring water pollution levels or soil pH.
- Food and Beverage: Determining the acidity in food items like wine and cheese.
- Research and Development: Developing new chemical compounds.
Volumetric Titration
Core Concept
Volumetric titration, often referred to as titrimetry, involves measuring the volume of titrant required to complete a chemical reaction with the analyte. The endpoint is typically indicated by a color change, thanks to an indicator dye that changes color at or near the endpoint.
Key Equipment
Key tools and equipment used in volumetric titration include:
- Burette: A precise measuring tool to deliver the titrant.
- Flask: Contains the solution to be titrated.
- Indicator: A substance that changes color at the endpoint.
Procedure Overview
- Fill the burette with a known concentration of titrant.
- Add the analyte to a flask along with a few drops of indicator.
- Gradually add titrant to the analyte, swirling to mix.
- Stop adding titrant when the color changes.
Advantages
- Cost-effective: Minimal equipment is needed.
- Simple setup: Easy for beginners to understand and perform.
- Wide applicability: Useful in many types of chemical analysis.
Limitations
- Subjectivity: Color change might be difficult to observe.
- Accuracy: Dependent on precise measurement and operation.
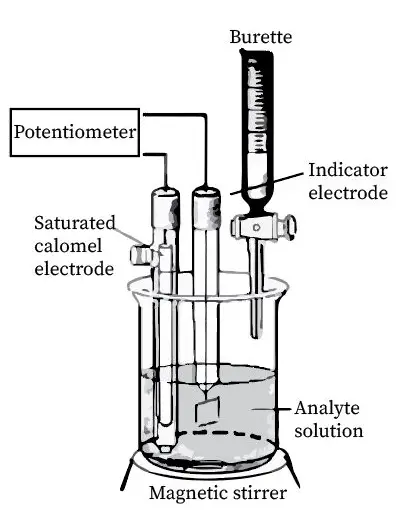
Potentiometric Titration
Core Concept
Potentiometric titration measures the change in electrical potential of the solution to determine the endpoint. It involves the use of electrodes to sense the voltage change, offering a more precise endpoint determination without the need for visual cues.
Key Equipment
- Electrode: Sensitive to specific ions in the solution.
- Potentiometer or pH meter: Records the voltage changes.
- Burette: Delivers the titrant.
Procedure Overview
- Set up the potentiometer or pH meter with the electrode.
- Add the analyte to a flask.
- Begin adding the titrant while continuously measuring the voltage.
- Determine the endpoint by noticing a stable or sharp change in voltage.
Advantages
- Precision: Highly accurate endpoint detection.
- Non-visual: Effective for colored or opaque solutions.
- Automation: Easily automated for consistent results.
Limitations
- Cost: More expensive equipment required.
- Complexity: Higher skill level needed for operation.
- Maintenance: Electrodes need regular calibration and maintenance.
Comparison and Contrast
Accuracy and Precision
Both volumetric and potentiometric titrations are fundamental in ensuring high accuracy and precision in chemical analysis. Volumetric titration generally offers reliable accuracy, especially in clear solutions where the endpoint can be visually determined. The precision of this method hinges on the skill of the operator to read the meniscus level accurately and consistently.
Potentiometric titration, however, provides superior precision. This method does not rely on visual cues, thus eliminating subjective interpretations of the endpoint. The use of electrodes to measure voltage changes directly linked to the concentration changes in the solution provides precise readings that are highly reproducible.
Sensitivity to Endpoints
Endpoint sensitivity is crucial in titration, as it determines how accurately the endpoint of a titration is detected. Volumetric titration relies on color indicators, which may not be effective for all types of reactions or in solutions where color changes are subtle. Potentiometric titration excels in this area, as it can detect minute changes in electric potential, making it ideal for reactions where the endpoint is not visually obvious.
Applicable Scenarios
The choice between volumetric and potentiometric titration often depends on the specific requirements of the experiment:
- Volumetric titration is preferred for routine analyses where speed and cost-efficiency are prioritized.
- Potentiometric titration is suited for complex matrices or where high precision is necessary.
Cost Effectiveness
In terms of cost, volumetric titration is more budget-friendly, involving less expensive equipment. It is a viable option for educational institutions and small-scale laboratories. Potentiometric titration, though more costly upfront due to the sophisticated equipment required, offers long-term cost savings through reduced reagent use and higher accuracy, minimizing the need for repeated experiments.
Practical Applications
Industry Use Cases
Titration methods are widely applied in various industries:
- Pharmaceuticals: Both methods are used to ensure the correct formulation and compliance with stringent quality controls.
- Environmental: Potentiometric titration is crucial for analyzing water pollutants at very low concentrations.
- Food and Beverage: Volumetric titration helps in determining food safety parameters like acidity and preservative levels.
Academic and Research Applications
In academic settings, titration serves as a key tool for teaching fundamental chemical principles and for conducting advanced research that requires precise quantitative analysis. Researchers rely on both types of titration to develop new materials, understand biological processes, and improve industrial chemicals.
Choosing the Right Method
Factors to Consider
Selecting the appropriate titration method involves considering several factors:
- Nature of the sample: Visibility, purity, and chemical properties.
- Required accuracy: Degree of precision necessary for the analysis.
- Available resources: Budget constraints and equipment availability.
Recommendations Based on Sample and Objective
For colorless and transparent samples where high accuracy is not critical, volumetric titration is often adequate. In contrast, for samples that are colored, opaque, or require stringent control of experimental conditions, potentiometric titration is recommended.
Innovations and Advances
Recent Developments in Titration Methods
Recent advances in titration focus on enhancing the precision, automation, and versatility of the methods. Automated titration systems have become more accessible, providing consistent results and reducing human error. Digital burettes and advanced sensors have also improved the efficiency and accuracy of both volumetric and potentiometric titrations.
Future Trends in Titration Techniques
The future of titration lies in the integration of IoT technologies and artificial intelligence. These technologies promise to further automate the process, allowing for real-time data analysis and adjustments. Innovations such as microfluidic systems and miniaturized sensors could lead to portable titration devices that are capable of performing complex analyses on-site.
Frequently Asked Questions
What is titration?
Titration is a method used in chemistry to determine the concentration of a substance in a solution. It involves the gradual addition of a solution of known concentration (the titrant) to a volume of another solution until the chemical reaction between the components is complete, indicated by a measurable change in the solution.
How does volumetric titration work?
In volumetric titration, a known concentration of titrant is added to a solution until the reaction reaches the endpoint, which is usually indicated by a color change. This change is often achieved with the help of a color indicator added to the solution, making it visible when the reaction is complete.
What is potentiometric titration?
Potentiometric titration involves measuring the electric potential or voltage of the solution to determine the endpoint of a titration. An electrode is inserted into the solution, and the potential change is monitored as the titrant is added, pinpointing the completion of the reaction without the need for a visual indicator.
When to use volumetric vs potentiometric titration?
The choice between volumetric and potentiometric titration depends on the specific requirements of the analysis. Volumetric titration is often preferred for its simplicity and cost-effectiveness in routine analyses, while potentiometric titration provides greater precision and is better suited for colored or opaque solutions where visual indicators would not be effective.
What are the advantages of potentiometric titration?
Potentiometric titration offers high accuracy and sensitivity, making it ideal for detecting the end of a reaction without subjective interpretation of color changes. It is especially useful in automated processes and where the solution’s clarity or color might obscure visual indicators.
Conclusion
Titration remains a cornerstone of quantitative analytical chemistry, with its methods continually refined to meet the evolving demands of science and industry. Understanding the distinct capabilities and limitations of volumetric and potentiometric titration not only aids in selecting the appropriate method for specific tasks but also enhances the accuracy and reliability of the results.
By appreciating the technical nuances and practical applications of these titration methods, researchers and professionals can ensure the precision of their analyses and the integrity of their conclusions. As the field of chemistry advances, the ongoing development and optimization of titration techniques will play a crucial role in maintaining the relevance and efficacy of this indispensable analytical tool.