Valency and oxidation number are two important concepts in chemistry that are often used interchangeably, but they actually have very distinct meanings. In this blog post, we’ll explore the key differences between valency and oxidation number and how they are related.
Definition of valency

Valency is the ability of an atom or molecule to combine with other elements or molecules. It is a measure of how many bonds can be formed by an atom in a molecule. Valency is related to the number of electrons available for bonding, and it is determined by the number of electrons surrounding an atom.
Valency is related to the number of electrons available for bonding, and it is determined by the number of electrons surrounding an atom. Valency is different from the oxidation number, which is a measure of the oxidation state of an atom. The oxidation number is determined by the number of electrons that have been removed or added to an atom.
The oxidation number is used to identify the species of an atom in a chemical reaction, while the valency is used to determine the type of bonding that will occur between atoms.
Definition of oxidation number
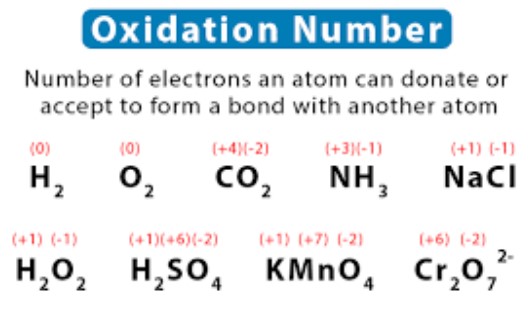
Oxidation number (also known as oxidation state) is an important concept in chemistry that describes the degree of oxidation of an atom in a chemical compound. It is a number assigned to each atom in a molecule to indicate its degree of oxidation, and it helps chemists keep track of electrons during chemical reactions.
Valency is the number of electrons an atom holds in its outermost shell, while oxidation number is the number of electrons an atom has lost or gained in a chemical reaction. For example, the oxidation number of oxygen is usually -2, while its valency is
Difference between valency and oxidation number

Valency and oxidation number are two concepts that are often confused and used interchangeably, but they have distinct meanings. Valency refers to the combining power of an element, while oxidation number reflects the charge of an atom in a compound. In other words, valency is a measure of how many atoms an element will bond with, while oxidation number is the charge an atom has when it is part of a compound.
Valency is determined by the number of electrons an element has in its outer shell, and usually increases with atomic number. Oxidation number, on the other hand, is assigned to each atom in a compound according to predetermined rules.
The oxidation number of an atom can be positive, negative, or zero. The main difference between valency and oxidation number is that oxidation number is a measure of the charge of an atom, while valency reflects the combining power of an element. Valency is determined by the number of electrons in an atom’s outer shell, while oxidation number is assigned according to predetermined rules.
Valency is determined by the number of electrons in an atom’s outer shell, while oxidation number is assigned according to predetermined rules.
Examples of valency and oxidation number
Valency and oxidation number are two concepts that are often confused when discussing chemical reactions. Valency is the number of electrons needed to complete an atom’s outermost shell, whereas oxidation number is the number of electrons an atom has gained or lost in a chemical reaction.
A key difference between the two is that while the valency of an element is fixed, the oxidation number can change during a reaction. For example, oxygen has a valency of 2, but its oxidation number can be -2, 0 or +2 depending on the reaction. Oxidation and reduction reactions are especially good examples of how oxidation number changes during a reaction.
An atom that has lost electrons has an oxidation number greater than its valency, while an atom that has gained electrons has an oxidation number less than its valency.
Benefits of knowing the difference between valency and oxidation number
When it comes to chemistry, it’s important to understand the distinction between valency and oxidation number. Valency is a measure of the ability of an atom to form chemical bonds with other atoms.
Oxidation number, on the other hand, is a measure of the overall charge of an atom in a molecule or polyatomic ion. Knowing the difference between these two concepts can help you better understand and predict the behavior of elements and compounds. Valency is determined by looking at the number of electrons in the outermost shell of an atom, while oxidation number is determined by looking at the overall charge of the atom.
By differentiating between these two concepts, you can gain a better understanding of chemical reactions and how atoms interact with each other.
Bottom Line
In conclusion, there is an important distinction between valency and oxidation number. Valency refers to the number of bonds an atom can form with another atom, while oxidation number is a measure of the charge of an atom in a given species.
Valency is determined by the number of electrons an atom has available to form bonds, while oxidation number is determined by the number of electrons an atom has gained or lost. Both valency and oxidation number are important concepts in chemistry and understanding the differences between them can help you better understand chemical reactions.