Chemical reactions are pivotal in both academic studies and practical applications, spanning from simple classroom experiments to complex industrial processes. Titration and neutralization, though often mentioned in the same breath, serve distinct purposes and embody unique methodologies within the realm of chemistry. By understanding their differences, one gains a clearer view of their applications and significance.
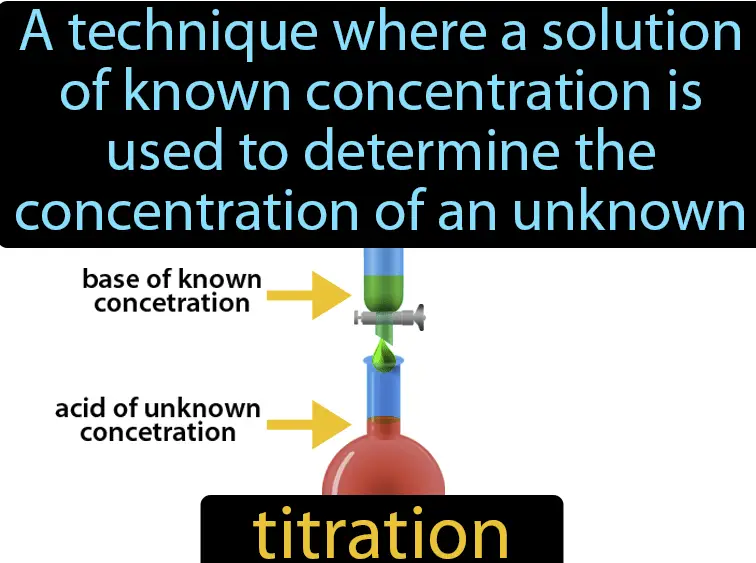
Titration is a quantitative analytical technique used to determine the concentration of an unknown solution by adding a solution of known concentration until the reaction reaches a completion point, often indicated by a color change. Neutralization, on the other hand, involves combining an acid and a base to form water and a salt, typically occurring when the acid and base are of equal strength and effectively cancel each other out.
Both titration and neutralization are fundamental in various scientific and industrial settings. Titration is crucial for quality control in pharmaceuticals, while neutralization plays a key role in managing industrial waste and environmental conservation. Understanding their specific roles enhances our ability to utilize these processes to their fullest potential.
Titration Explained
Definition and Purpose
Titration is a fundamental process in analytical chemistry that involves the gradual addition of one solution of a known concentration (the titrant) to another substance or solution of unknown concentration. The main purpose of titration is to determine the concentration of an unknown sample accurately. This method is pivotal in various fields such as environmental science, medicine, and food science, offering precise and critical data that influence quality control and safety regulations.
Common Types of Titration
Acid-base Titration
Acid-base titration is one of the most common types of titration conducted in laboratories. It involves the process of adding a base solution to an acid solution or vice versa. The endpoint of this titration is typically determined by a pH indicator that changes color at a certain pH value. This type of titration is essential for determining the concentrations of unknown acidic or basic compounds in a solution.
Redox Titration
Redox titration is another critical type of titration that involves a reduction-oxidation reaction between the analyte and the titrant. The endpoint of redox titrations can be detected by an indicator that changes color when a specific amount of reducing or oxidizing agent has been added. This method is commonly used in determining the purity of water and the concentration of metals in various solutions.
Complexometric Titration
Complexometric titration is primarily used for finding the concentration of metal ions in a solution. This technique uses a complexing agent that forms a very stable complex with the metal ions. An indicator, which can form a weaker complex with the metal ions, is then used to show the endpoint of the titration. This type is essential for water hardness testing and measuring the metal content in pharmaceutical products.
Neutralization Basics
What is Neutralization?
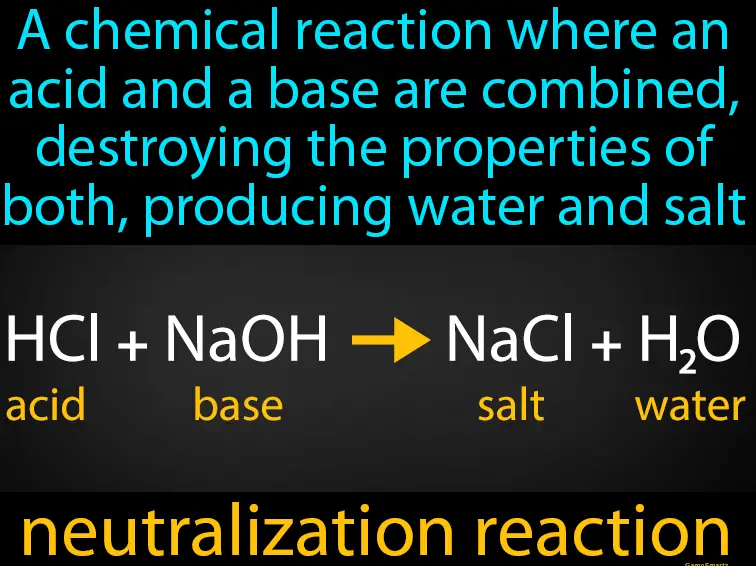
Neutralization is a chemical reaction where an acid and a base react to form water and a salt. This reaction is fundamental in various chemical processes and plays a critical role in maintaining environmental and industrial safety. Neutralization helps in balancing pH levels in wastewater and other industrial effluents, preventing damage to ecosystems and machinery.
Role in Everyday Chemistry
Neutralization reactions are not just confined to laboratories; they are part of everyday life. From treating a bee sting with baking soda (a base) to neutralize the venom’s acidity, to taking antacids to alleviate stomach acidity, these reactions are ubiquitous. In environmental management, neutralization is used to treat acid rain effects and manage soil acidity, enhancing agricultural productivity.
Process Comparison
Titration Steps
- Preparation of Solutions: Ensure all solutions are prepared accurately, with the titrant of known concentration precisely measured.
- Setup: Arrange the apparatus, typically including a burette, to facilitate the controlled addition of the titrant.
- Indicator Addition: Add the appropriate indicator to the analyte solution.
- Titration: Gradually add the titrant to the analyte until the endpoint is reached, indicated by a color change.
- Calculation: Calculate the unknown concentration using the volume of titrant used and its known concentration.
Neutralization Steps
- Mixing Reactants: Combine measured amounts of acid and base, depending on the desired reaction scale.
- Monitoring pH: Continuously monitor the pH of the reaction mixture to approach the neutral point without overshooting.
- Endpoint Detection: Determine the endpoint either by pH meter or using an indicator that signals when neutralization is achieved.
- Disposal/Processing: Safely dispose of or process the resultant mixture, typically a neutral salt solution.
Equipment and Materials
Tools for Titration
The essential tools for titration include:
- Burette: Precision instrument for dispensing the titrant.
- Pipette: For measuring and transferring the analyte.
- Conical Flask: To mix solutions during titration.
- pH Meter/Indicator: To determine the endpoint of the titration.
Materials for Neutralization
Materials needed for effective neutralization include:
- Acids and Bases: Specific to the reaction requirements.
- Indicator: To visually or electronically determine the endpoint of neutralization.
- Protective Gear: Safety glasses and gloves to handle corrosive substances safely.
Applications
Industrial Uses of Titration
Titration plays a critical role in various industries, ensuring product quality and compliance with safety standards. For instance, in the pharmaceutical industry, titration is used to determine the concentration of active ingredients in drugs, ensuring each batch meets the required potency for effective treatment. In the food and beverage sector, titration helps in analyzing food additives and preservative levels, crucial for food safety and regulatory compliance. Furthermore, the chemical manufacturing industry relies on titration for process control, ensuring reactions have reached their completion and optimizing the use of raw materials.
Everyday Examples of Neutralization
Neutralization reactions are commonplace in everyday life, often observed without realization. Common examples include:
- Cooking: Baking powder, which contains sodium bicarbonate (a base), reacts with moisture and acidic ingredients to release carbon dioxide, helping dough rise.
- Healthcare: Antacids like calcium carbonate neutralize stomach acid, providing relief from heartburn and acid reflux.
- Agriculture: Farmers use lime (calcium carbonate) to neutralize acidic soils, improving fertility and crop yields.
Accuracy and Precision
Precision in Titration
Precision in titration is vital, as it dictates the reliability of the analysis. High precision in titration is achieved through:
- Consistent Technique: Standardizing how the titrant is added to the analyte.
- Calibration of Equipment: Regularly calibrating pipettes, burettes, and pH meters to avoid deviation.
- Reproducibility: Performing multiple titrations to ensure consistent results across trials.
Accuracy in Neutralization
In neutralization, accuracy ensures the complete and effective reaction between acids and bases. This is critical in processes like waste water treatment, where accurate pH adjustments prevent environmental harm. Accuracy can be improved by:
- Precise Measurement: Using calibrated instruments to measure the quantities of reactants.
- Controlled Conditions: Monitoring temperature and reaction conditions to maintain consistent environmental factors.
Calculation Methods
Formulas in Titration
The fundamental formula used in titration to determine the concentration of an unknown solution is: 𝐶1𝑉1=𝐶2𝑉2C1V1=C2V2 Where 𝐶1C1 and 𝐶2C2 are the concentrations of the solutions, and 𝑉1V1 and 𝑉2V2 are the volumes of the titrant and analyte. This formula is pivotal in calculating the exact concentration of the unknown substance when the other variables are known.
Equations for Neutralization
The general equation for a neutralization reaction is: Acid+Base→Water+SaltAcid+Base→Water+Salt This simplified representation underscores the direct relationship between acids and bases leading to salt and water, which is crucial in formulating products like antacids and in industrial processes like wastewater treatment.
Environmental Impact
Titration Waste Management
Proper disposal of waste generated from titration processes is essential to minimize environmental impact. This includes:
- Neutralization of Wastes: Before disposal, acidic or basic wastes are neutralized to prevent harm to water sources.
- Recycling of Solvents: Where possible, solvents used in titration are recycled, reducing the need for fresh chemicals and minimizing waste.
Neutralization and the Environment
Neutralization reactions are beneficial for environmental management. They play a crucial role in:
- Treating Acid Rain: Neutralizing acidic water bodies affected by acid rain to protect aquatic life.
- Industrial Effluent Treatment: Neutralizing industrial effluents before they are discharged into the environment, ensuring they meet environmental safety standards.
Safety Protocols
Safety in Titration Procedures
Safety during titration is paramount to prevent accidents and ensure the well-being of personnel. Key safety measures include:
- Use of Protective Gear: Wearing gloves, goggles, and protective clothing.
- Proper Handling of Chemicals: Training staff in the safe handling and disposal of hazardous chemicals.
Handling Chemicals during Neutralization
The handling of acids and bases during neutralization requires careful safety protocols to avoid accidents, including:
- Proper Ventilation: Ensuring that work areas are well-ventilated to dissipate harmful vapors.
- Emergency Measures: Having neutralizing agents and first aid readily available in case of spills or contact.
Frequently Asked Questions
What is Titration?
Titration is a laboratory method of quantitative chemical analysis used to determine the unknown concentration of an identified analyte. By adding a reagent of known concentration and volume to react with the analyte, titration helps in achieving the equivalence point where the reaction is complete.
How Does Neutralization Work?
Neutralization is a chemical reaction where an acid and a base react quantitatively with water and a salt as the products. This reaction is fundamental in various applications, including wastewater treatment where harmful acids or bases are neutralized.
Why is Titration Important in Industry?
Titration is crucial for industries as it ensures the safety and efficacy of products. Particularly in pharmaceuticals, food, and chemical manufacturing, titration helps in quality control by precisely measuring substance concentrations within a solution.
Can Neutralization Be Dangerous?
While neutralization is a generally safe process, handling strong acids and bases can be hazardous. Proper safety measures must be observed to prevent chemical burns and other injuries associated with corrosive substances.
Conclusion
Titration and neutralization, while both rooted in the basic principles of chemistry, cater to different scientific needs and applications. Titration offers a method to meticulously measure and analyze the concentration of chemicals, playing a critical role in research and industrial quality control. On the other hand, neutralization provides a straightforward solution to mitigate the effects of acidic or basic substances, essential for environmental management and safety protocols.
Recognizing the distinct characteristics and applications of titration and neutralization not only enriches our understanding but also highlights the versatility and importance of chemical reactions in daily and professional contexts. This knowledge empowers us to apply these processes more effectively in scientific exploration and various industrial practices.

