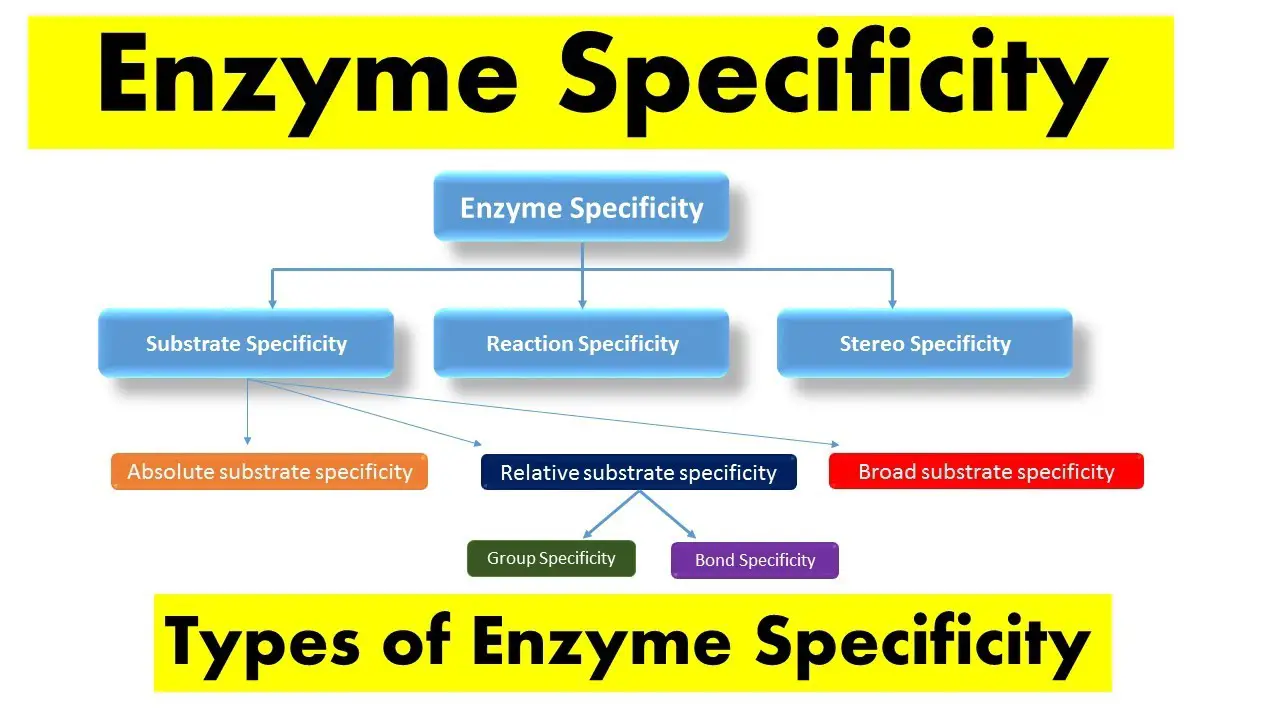
Enzyme specificity is a fundamental concept in biochemistry, critical for understanding how biological reactions are catalyzed. Enzymes, which are proteins that act as catalysts in the body, exhibit specificity — meaning they select specific substrates from a complex mixture of molecules to carry out chemical reactions. This specificity determines the efficiency and selectivity of metabolic pathways.
Substrate specificity refers to the ability of an enzyme to choose only one substrate out of many possible ones in a reaction, based on the shape and chemical makeup of the substrate. Bond specificity, on the other hand, relates to the enzyme’s ability to catalyze the reaction at a specific type of bond within the substrate, irrespective of the rest of the molecular structure. These two types of specificity play pivotal roles in the function and regulation of enzymes within the cell.
The difference between substrate specificity and bond specificity lies in their approach and impact on enzymatic actions. Substrate specificity is influenced by the three-dimensional structure of the enzyme and the substrate, while bond specificity focuses more on the type of chemical bond involved in the reaction. Understanding these differences not only sheds light on enzyme functionality but also has significant implications in drug design and biotechnology.
Substrate Specificity
Definition and Basics
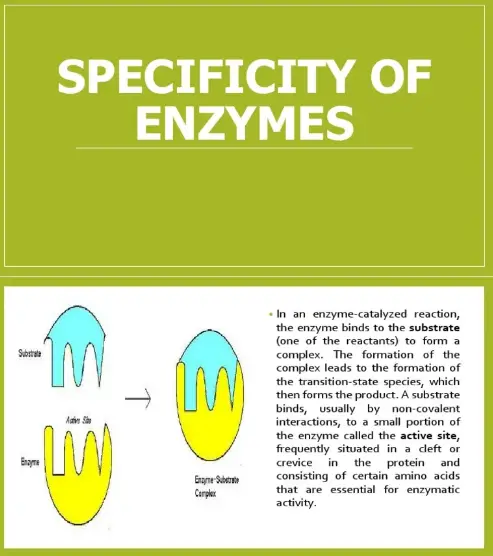
Substrate specificity is a key characteristic of enzymes, defining their ability to select and interact with a specific substrate among many similar molecules in the cell. An enzyme’s active site—the part of the molecule that binds to the substrate—is uniquely suited to one particular substrate, thanks to the precise shape and chemical properties it possesses. This specificity ensures that enzymes catalyze only the appropriate reactions necessary for cellular metabolism.
Role in Enzyme Function
Substrate specificity is critical for maintaining the efficiency and timing of metabolic processes. Without this specificity, enzymes could catalyze unwanted reactions, leading to metabolic chaos and harmful cellular conditions. For example, the enzyme hexokinase shows high substrate specificity as it primarily catalyzes the phosphorylation of glucose, a crucial step in the metabolic pathway of glycolysis. This specificity ensures that glucose is utilized efficiently in energy production, rather than being redirected into other less critical pathways.
Examples in Biological Systems
In biological systems, substrate specificity plays a vital role in regulating pathways and maintaining cellular health:
- Digestive enzymes: Amylase in saliva specifically breaks down starch into simpler sugars but does not act on proteins or fats.
- DNA polymerases: These enzymes are highly specific in recognizing the nucleotide sequence of a DNA strand to ensure accurate DNA replication and repair.
- Hormone-sensitive lipase: This enzyme in fat cells selectively hydrolyzes triglycerides into free fatty acids and glycerol, a process that is crucial during energy demand.
Bond Specificity
Definition and Explanation
Bond specificity refers to the preference of an enzyme to catalyze reactions at specific types of chemical bonds, regardless of the larger structure of the substrate. This type of specificity is less about the overall molecular fit and more about the enzyme’s ability to recognize and act upon a particular bond within diverse substrates.
How It Differs from Substrate Specificity
Unlike substrate specificity, bond specificity does not require a unique three-dimensional structure match between the enzyme and the entire substrate molecule. Instead, enzymes with bond specificity focus on a specific bond type. For instance, a protease with bond specificity might cleave peptide bonds specifically, whether they are found in small peptides or large protein complexes.
Key Examples and Applications
Bond specificity has important applications in both nature and industry:
- Thrombin: This enzyme plays a critical role in blood clotting by specifically cleaving the peptide bonds in fibrinogen to form fibrin, the primary protein of blood clots.
- Industrial meat tenderizers: These contain papain, an enzyme that breaks down specific peptide bonds in muscle protein, making the meat tender.
Comparative Analysis
Direct Comparison of Substrate and Bond Specificity
Substrate specificity and bond specificity both guide the action of enzymes but in markedly different ways. Substrate specificity ensures that only specific substrates are transformed, guided by the structural compatibility. In contrast, bond specificity allows for a broader range of substrate interaction but limits the reaction to specific types of chemical bonds.
Table: Differences at a Glance
| Aspect | Substrate Specificity | Bond Specificity |
|---|---|---|
| Focus | Entire substrate molecule | Specific type of bond within a substrate |
| Applications | Metabolic pathways, drug design | Blood clotting, meat processing |
| Biological Role | Ensures metabolic integrity and efficiency | Facilitates reactions in diverse contexts |
Factors Influencing Specificity
Chemical Structure
The chemical structure of both the enzyme and the substrate significantly impacts specificity. The active site of the enzyme is often a mirror image of the substrate or part of it, allowing precise binding and catalysis.
Environmental Factors
pH levels, temperature, and ionic strength of the environment can alter enzyme conformation and thus affect specificity. Enzymes are adapted to their operational environment, and any deviation can reduce their efficiency or change their specificity.
Evolutionary Considerations
Evolutionary pressures have shaped enzymes to develop and maintain specificity that benefits the organism’s survival and efficiency. Variations in enzyme specificity among species often reflect adaptations to different environmental conditions or dietary needs.
Impact on Drug Design
Substrate Specificity in Pharmaceuticals
Substrate specificity is pivotal in the development of pharmaceutical drugs as it allows for the design of molecule-targeted therapies that can interact precisely with specific enzymes associated with diseases. This precision not only enhances the drug’s effectiveness but also minimizes side effects by reducing unintended interactions with other enzymes or receptors.
- Selective enzyme inhibitors: Drugs designed to block the activity of specific enzymes involved in disease processes. For example, protease inhibitors used in treating HIV are tailored to block the virus-specific enzyme, preventing viral replication without affecting human enzymes.
- Prodrug activation: Some drugs are designed as inactive prodrugs that are metabolized into active drugs only in the presence of specific enzymes found in target tissues or cells. This strategy reduces the systemic side effects of the drugs.
Bond Specificity in Targeted Therapies
Bond specificity is crucial in designing therapies that need to act on specific types of chemical bonds within the pathological molecules. By focusing on bond types, treatments can be more tailored and effective in disrupting the molecular pathways that contribute to disease.
- Antibody-drug conjugates (ADCs): These are targeted cancer therapies designed to deliver cytotoxic agents specifically to tumor cells. The linker between the antibody and the drug is designed to be cleaved by enzymes that recognize specific bonds, ensuring that the drug is activated only when it reaches the cancer cells.
Technological Applications
Biotechnology and Genetic Engineering
In biotechnology and genetic engineering, enzyme specificity is exploited to modify genes and proteins with high precision. Techniques such as CRISPR-Cas9 gene editing utilize the enzyme’s specificity for identifying and cutting DNA at specific sequences, leading to advancements in genetic research and therapy.
- Genome editing: Specificity in CRISPR systems allows for precise modifications at targeted locations in the genome, enabling the treatment of genetic disorders by correcting mutations at their source.
- Protein engineering: Enzymes are modified to improve their specificity, making them more efficient for industrial applications, including pharmaceutical manufacturing and environmental remediation.
Industrial Enzyme Applications
The specificity of enzymes is also harnessed in various industrial processes to enhance efficiency and sustainability.
- Biofuel production: Enzymes with specificity for certain plant cell wall components are used to break down biomass into sugars, which are then converted into biofuels.
- Textile industry: Specific enzymes are used in processing fabrics, such as cellulases for giving jeans a stone-washed look without using harsh chemicals.
Future Perspectives
Research Trends
Current research in enzyme specificity focuses on understanding how enzymes evolve and adapt their specificity, which can lead to the discovery of new enzymes or the redesign of existing ones for better functionality. Advances in computational biology and structural biochemistry play significant roles in these developments.
- Synthetic biology: Designing new enzymes with tailor-made specificities for use in novel reactions, potentially transforming chemical manufacturing and therapeutic development.
Potential Innovations in Specificity
The future of enzyme specificity in biotechnology and medicine includes several promising areas:
- Smart drugs: Development of drugs that can change their specificity in response to biofeedback, providing dynamic treatment options.
- Enzyme inhibitors: New classes of inhibitors that can modulate enzyme activity with unprecedented specificity, offering new ways to treat diseases that have been resistant to other forms of therapy.
Frequently Asked Questions
What is enzyme specificity?
Enzyme specificity is the ability of an enzyme to choose exactly one substrate from a mixture of molecules and catalyze a particular reaction. This selectivity ensures that enzymes catalyze only the correct reactions, which is essential for proper metabolic function.
How does substrate specificity differ from bond specificity?
Substrate specificity is determined by the overall structure of the substrate molecule and the enzyme’s active site, ensuring that only a particular substrate can bind. Bond specificity, however, depends on the type of chemical bond to be acted upon, regardless of the substrate’s overall structure.
Why is enzyme specificity important in pharmaceuticals?
Enzyme specificity is crucial in pharmaceuticals because it allows for the design of drugs that can target specific enzymes linked to a disease. By understanding and exploiting enzyme specificity, medications can be developed to interact precisely with their target, minimizing side effects and enhancing efficacy.
What role does bond specificity play in industrial applications?
Bond specificity is vital in industrial applications such as the synthesis of biofuels and the processing of textiles. Enzymes selected for their bond-specific actions can break down or synthesize chemical bonds efficiently, leading to more sustainable and cost-effective industrial processes.
Conclusion
The distinction between substrate specificity and bond specificity is not just an academic detail but a cornerstone in the development of targeted therapies and efficient biocatalysts. By understanding these differences, researchers and engineers can harness the full potential of enzymes in medicine and industry.
The exploration of enzyme specificity opens doors to innovative solutions in drug design and biotechnological applications, highlighting the importance of detailed biochemical knowledge in advancing science and technology.