Fluoride compounds play a pivotal role in modern dental health, specifically in the prevention of cavities and strengthening of tooth enamel. Two commonly used fluoride derivatives in dental products are Sodium Fluoride (NaF) and Sodium Monofluorophosphate (SMFP). Each serves a distinct purpose and possesses unique characteristics that contribute to oral health.
Sodium Fluoride is a simple inorganic compound used extensively in toothpaste and mouth rinses to help prevent dental caries. Sodium Monofluorophosphate, on the other hand, is a compound that releases fluoride ions in the presence of enzymes found in the mouth, offering a similar protection against tooth decay. Both compounds are effective, but their chemical properties and mechanisms of action differ, influencing their use in various dental care products.
The significance of these fluoride compounds extends beyond just their application in dental hygiene. They are integral to discussions about public health, environmental impact, and consumer choices, reflecting their broader relevance to societal and ecological considerations. Understanding their differences is crucial for making informed decisions about dental care products and for appreciating the nuances of fluoride use in dentistry.
Chemical Basics
What is Sodium Fluoride?
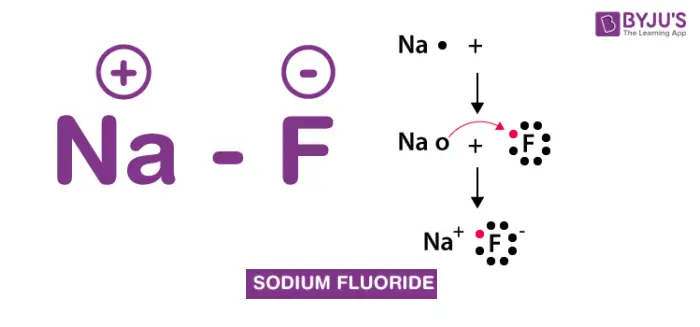
Sodium Fluoride (NaF) is an inorganic compound widely recognized for its use in dental care products due to its efficacy in preventing dental caries. It is a colorless solid that dissolves easily in water, making it ideal for use in various formulations. Sodium Fluoride directly supplies fluoride ions to the teeth when applied, which are crucial for the remineralization process that helps harden tooth enamel and reduces the risk of decay.
What is Sodium Monofluorophosphate?
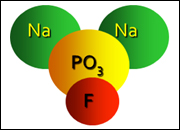
On the other hand, Sodium Monofluorophosphate (SMFP) also plays a significant role in dental health but differs chemically and functionally from Sodium Fluoride. It is an organic compound containing fluoride, and its structure allows it to release fluoride ions in the presence of water and enzymes found in saliva. This characteristic makes SMFP particularly effective in slow-release fluoride applications in dental hygiene products.
Historical Context
Discovery and Early Use
Sodium Fluoride was first used in the 19th century, but its importance in dental care was not realized until the early 20th century. Researchers noted that communities with naturally occurring fluoride in water had lower incidences of dental cavities. Sodium Monofluorophosphate was later developed and incorporated into dental products, providing an alternative that could be used with less direct exposure to fluoride but with similar benefits.
Evolution in Dental Products
Initially, Sodium Fluoride dominated as the fluoride agent of choice in dental care products. Over the years, as the understanding of fluoride’s benefits expanded, Sodium Monofluorophosphate began to be included in formulations, particularly in toothpastes and mouthwashes. This evolution was driven by the need for products that could deliver fluoride more safely and effectively, minimizing the risk of fluorosis while maximizing dental health benefits.
Chemical Properties
Structural Differences
The key difference between Sodium Fluoride and Sodium Monofluorophosphate lies in their molecular structure. Sodium Fluoride is a simple salt made up of sodium and fluoride ions, making it straightforward and quick in its action. In contrast, Sodium Monofluorophosphate features a more complex structure with a phosphate group, which affects how fluoride ions are released and utilized in the mouth.
Solubility and Stability
Sodium Fluoride is highly soluble in water, which facilitates its immediate availability to interact with tooth enamel upon application. Sodium Monofluorophosphate is slightly less soluble but provides a stable method of delivering fluoride over a longer period, which can be advantageous in daily oral hygiene routines.
Mechanism of Action
How Sodium Fluoride Works
Sodium Fluoride works by directly interacting with the minerals in tooth enamel. It helps to rebuild weakened enamel through a process called remineralization. The fluoride ions from Sodium Fluoride form a new, harder enamel layer that is more resistant to acid and bacterial attack, effectively helping to prevent the development of cavities.
How Sodium Monofluorophosphate Works
Sodium Monofluorophosphate requires enzymatic activity to release fluoride ions. When applied in the form of toothpaste or mouthwash, enzymes in the saliva break down the Monofluorophosphate compound, slowly releasing fluoride ions. This gradual process helps maintain a longer-lasting protective barrier against decay.
Dental Applications
Use in Toothpaste
Both Sodium Fluoride and Sodium Monofluorophosphate are integral to modern toothpaste formulations. They are selected based on their properties, with Sodium Fluoride often chosen for immediate fluoride release and Sodium Monofluorophosphate for its extended-release capabilities, catering to different consumer needs and preferences.
Use in Mouthwashes
In mouthwashes, the choice between Sodium Fluoride and Sodium Monofluorophosphate often depends on the desired effect and the formulation’s overall design. Mouthwashes with Sodium Fluoride provide a quick, intense exposure to fluoride, which is beneficial for immediate remineralization, while those with Sodium Monofluorophosphate offer a more gradual protective action, beneficial for long-term dental care regimes.

Health Implications
Benefits to Dental Health
Both Sodium Fluoride and Sodium Monofluorophosphate are celebrated for their preventive effects on dental caries, a major health issue globally. Fluoride’s primary benefit is its ability to remineralize tooth enamel, making it harder and more resistant to acid attacks from bacteria in the mouth. This is crucial in both preventing cavities and halting further decay in teeth already exhibiting signs of dental caries.
Regular use of fluoride-containing products can also contribute to a reduction in plaque formation, as fluoride creates a hostile environment for bacteria that cause plaque and gum disease. This double action—strengthening enamel and protecting against bacterial attack—underpins the widespread endorsement of fluoride by dental health professionals.
Potential Risks and Side Effects
While fluoride is highly beneficial, excessive exposure can lead to dental fluorosis, a condition marked by changes in the appearance of tooth enamel. This usually occurs when children, whose teeth are still developing, ingest too much fluoride. In severe cases, skeletal fluorosis can occur, affecting bones and joints.
It’s important to use fluoride products according to guidelines to avoid these risks. Moreover, individuals with specific health conditions or allergies should consult with healthcare providers before using fluoride-based products.
Environmental Impact
Effects on Ecosystems
The environmental impact of fluoride compounds, particularly when they enter freshwater ecosystems, is a significant concern. High levels of fluoride can be toxic to plants and animals, affecting reproduction and leading to biodiversity loss in aquatic environments. The mobility of fluoride ions in water makes it a persistent environmental pollutant in areas with high natural fluoride or from industrial sources.
Disposal and Management
Proper disposal of fluoride-containing products is essential to minimize their environmental footprint. This includes ensuring that toothpaste tubes and mouthwash bottles are disposed of correctly, and that industrial fluoride waste is treated and managed in accordance with environmental protection standards. Communities should promote the safe handling of these substances to prevent accidental spillages that could lead to soil and water contamination.
Comparative Analysis
Effectiveness in Cavity Prevention
Studies consistently show that both Sodium Fluoride and Sodium Monofluorophosphate are effective in preventing dental cavities, albeit through slightly different mechanisms of action. Sodium Fluoride is particularly efficient due to its direct interaction with tooth enamel, while Sodium Monofluorophosphate offers a sustained-release that keeps providing protection over a longer period.
Popularity and Usage Trends
The popularity of fluoride-based products has evolved over the years, influenced by public health policies, consumer preferences, and scientific advancements. Today, Sodium Fluoride remains a staple in many toothpastes and mouthwashes due to its immediate efficacy, while Sodium Monofluorophosphate is favored in products designed for sensitive teeth or where slow fluoride release is preferred.
Consumer Choices
How to Choose the Right Product
Choosing the right fluoride toothpaste or mouthwash can significantly impact dental health outcomes. Here are a few simple steps to guide consumer choices:
- Identify fluoride content: Check the label for active ingredients to ensure the product contains Sodium Fluoride or Sodium Monofluorophosphate.
- Consider dental needs: Choose a product based on specific dental needs such as cavity prevention, enamel protection, or sensitivity relief.
- Consult a dentist: Always consult with a dental care professional to select a product that best suits individual health profiles and dental conditions.
Label Reading and Understanding
Understanding labels is key to selecting effective fluoride products:
- Look for ADA approval: Products approved by the American Dental Association (ADA) meet strict safety and efficacy guidelines.
- Check fluoride concentration: Ensure the product has an appropriate level of fluoride suitable for all household members, especially children.
- Examine other active ingredients: Some products contain additional active ingredients for whitening, tartar control, or breath freshening, which may influence choice based on personal preferences or needs.
Frequently Asked Questions
What is Sodium Fluoride?
Sodium Fluoride (NaF) is an inorganic, colorless compound used primarily to prevent dental cavities in both children and adults. It strengthens tooth enamel and increases resistance to bacterial degradation.
What is Sodium Monofluorophosphate?
Sodium Monofluorophosphate (SMFP) is a fluoride compound commonly used in toothpastes to protect against dental decay. It releases fluoride ions when metabolized by enzymes in the mouth, which are then available to help remineralize and strengthen tooth enamel.
How do Sodium Fluoride and Sodium Monofluorophosphate differ in their action?
While both compounds ultimately aid in tooth enamel strengthening and cavity prevention, Sodium Fluoride acts directly by releasing fluoride ions that integrate into the tooth enamel. Sodium Monofluorophosphate requires enzymatic action to release the fluoride ions, making its effect slightly delayed compared to Sodium Fluoride.
Are there any risks associated with using Sodium Fluoride or Sodium Monofluorophosphate?
Both compounds are considered safe for use in dental products when used as directed. However, excessive intake of fluoride can lead to dental fluorosis or skeletal fluorosis, which are conditions caused by fluoride overexposure and are more common in areas with naturally high fluoride levels in water.
Conclusion
The distinction between Sodium Fluoride and Sodium Monofluorophosphate is more than just chemical; it affects how each is utilized in dental care products and how they interact with the human body. By choosing the appropriate fluoride-based product, consumers can effectively contribute to their oral health while minimizing potential risks.
The ongoing development and research into these compounds will likely continue to refine their uses and effectiveness in dental care. As consumers, staying informed about these advancements is crucial for making educated decisions regarding our health and well-being.