Cyanides, notorious for their toxicity, play a pivotal role in various industrial applications, primarily in mining and chemical synthesis. The mention of cyanide often conjures images of danger and mortality; however, its utility in industrial processes cannot be overstated. Sodium cyanide and potassium cyanide, two compounds within this classification, serve as the backbone for numerous chemical processes, each possessing unique characteristics and uses.
The primary difference between sodium cyanide and potassium cyanide lies in their chemical composition and physical properties. Sodium cyanide, denoted as NaCN, and potassium cyanide, indicated as KCN, differ in solubility, toxicity, and application areas, making each suitable for specific industrial tasks. Despite their similarities in appearance and potential hazards, understanding their distinct attributes is crucial for safe and effective usage.
These compounds are critical in metal processing, especially in extracting precious metals like gold and silver. Sodium cyanide, preferred for its cost-effectiveness and efficiency in the gold leaching process, contrasts with potassium cyanide, which finds its application in organic synthesis and electroplating. Both chemicals, while invaluable to their respective industries, pose significant environmental and health risks, necessitating stringent handling and disposal practices.
Chemical Properties
Composition and Structure
Differences in Atomic Composition
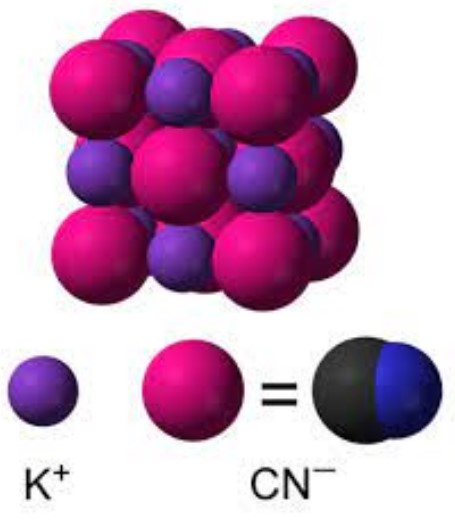
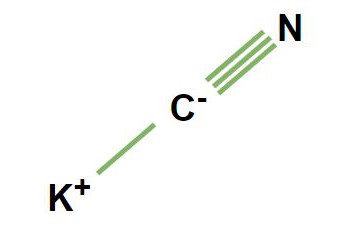
Sodium cyanide (NaCN) and potassium cyanide (KCN) are chemical compounds that differ primarily in their atomic composition. The former contains sodium (Na), while the latter comprises potassium (K) as the metal ion. This distinction influences their properties and applications. Both compounds are composed of a cyanide group (CN), which is a carbon (C) atom triple-bonded to a nitrogen (N) atom, but the metal ion’s nature—sodium vs. potassium—alters their behavior in various contexts.
Molecular Structure Comparison
The molecular structures of NaCN and KCN are similar, with the cyanide ion (CN-) bonded to either a sodium or potassium ion. This simple structure is crucial for their reactivity and applications. Despite this similarity, the ionic radii and electronegativity of sodium and potassium differ, affecting the solubility, reactivity, and toxicity of these compounds.
Reactivity and Stability
Reactivity with Water and Acids
Both sodium cyanide and potassium cyanide are highly reactive with acids, releasing hydrogen cyanide gas (HCN), a highly toxic substance. Their reactivity with water is less pronounced but still significant, especially when it leads to the slow release of HCN in moist air.
Thermal Stability Differences
Sodium cyanide has a melting point of approximately 563°F (295°C), while potassium cyanide has a lower melting point of about 634°F (335°C). This indicates that KCN is somewhat more thermally stable than NaCN, which can be an important consideration in processes involving high temperatures.
Physical Properties
Appearance and Form
Color and Physical State
Both sodium cyanide and potassium cyanide appear as white crystalline solids. Their physical form and color do not significantly differ, making it difficult to distinguish between them without chemical analysis.
Solubility in Water
Sodium cyanide is slightly more soluble in water than potassium cyanide. This property is particularly relevant in mining applications, where the solubility of cyanide compounds in water is utilized to extract precious metals from ore.
Toxicity Levels
LD50 Comparison
The lethal dose 50 (LD50), which is the dose lethal to 50% of a test population, varies slightly between these compounds, though both are extremely toxic. The LD50 for sodium cyanide is slightly lower than for potassium cyanide, indicating marginally higher toxicity.
Mechanism of Toxicity
The toxicity of both compounds arises from their ability to inhibit cytochrome c oxidase, a critical enzyme in the body’s mitochondrial electron transport chain. This inhibition blocks cellular respiration, leading to death from histotoxic hypoxia.
Industrial Uses
Mining and Metal Processing
Role in Gold and Silver Extraction
Sodium cyanide is extensively used in the mining industry for the extraction of gold and silver from ore. This process, known as cyanidation, leverages the compound’s solubility and reactivity to efficiently leach precious metals from rock.
Differences in Efficiency and Preference
The preference for sodium cyanide in gold and silver mining is primarily due to its greater efficiency and cost-effectiveness. Though potassium cyanide can be used similarly, its higher cost and slightly different reactivity profile make it less common for this purpose.
Chemical Synthesis
Applications in Organic Synthesis
Potassium cyanide is often preferred in organic synthesis applications, where it serves as a source of the cyanide ion for nucleophilic addition reactions, among other uses. Its reactivity is suitable for the synthesis of nitriles and other organic compounds.
Comparison in Pharmaceutical Manufacturing
In pharmaceutical manufacturing, potassium cyanide’s utility in synthesizing a variety of active pharmaceutical ingredients (APIs) showcases its importance. While sodium cyanide can also be used, the choice between them depends on the specific chemical reactions required for API synthesis.
Environmental Impact
Toxicity to Aquatic Life
Both sodium and potassium cyanide pose significant risks to aquatic life, with the potential to cause massive fish kills and disrupt aquatic ecosystems. Their high toxicity affects both freshwater and marine environments.
Degradation Rates in Water
Cyanides degrade in water through natural processes, such as hydrolysis and photolysis, albeit slowly. These degradation rates are critical in assessing the long-term environmental impact of cyanide spills or leaks.
Disposal and Treatment
Approved Disposal Methods
Safe disposal of sodium and potassium cyanide involves neutralization to less harmful chemicals or incineration under controlled conditions. These methods are designed to mitigate environmental and health risks.
Challenges in Cyanide Waste Treatment
Treating cyanide waste poses significant challenges, primarily due to the compounds’ toxicity and the need for specialized equipment and techniques. Ensuring that disposal methods effectively neutralize or destroy the cyanide without releasing harmful byproducts requires careful planning and regulation.

Safety Measures
Handling and Storage
Safety Protocols for Industrial Handling
Handling sodium cyanide and potassium cyanide requires strict safety protocols due to their high toxicity. Key guidelines include:
- Use of personal protective equipment (PPE), such as gloves, goggles, and respirators.
- Ensuring adequate ventilation in areas where cyanides are handled.
- Implementing spill containment measures to prevent environmental contamination.
Training for workers on the risks associated with cyanide exposure and the correct response to spills or exposure is essential.
Storage Requirements Differences
Storage practices for sodium and potassium cyanide differ slightly due to their physical properties. Key points include:
- Both chemicals should be stored in cool, dry, well-ventilated areas away from acids.
- Potassium cyanide, being slightly more hygroscopic (absorbs moisture from the air), requires tighter moisture control.
- Containers must be clearly labeled and made of materials that do not react with cyanides.
Emergency Response
First Aid Measures
In case of exposure to cyanides, immediate actions include:
- Inhalation: Move the victim to fresh air immediately. Administer oxygen if breathing is difficult.
- Skin/Eye Contact: Flush affected area with plenty of water for at least 15 minutes.
- Ingestion: Do not induce vomiting. Seek immediate medical attention.
Antidotes and Treatment Options
Hydroxocobalamin is an effective antidote for cyanide poisoning, as it binds to cyanide ions, forming a non-toxic complex that is excreted from the body. Other treatments include oxygen therapy and the use of sodium thiosulfate, which aids in the detoxification process.
Cost and Availability
Production Costs
The production costs of sodium and potassium cyanide depend on several factors:
- The price of raw materials, such as sodium or potassium and carbon sources.
- Energy costs associated with the manufacturing process.
- Environmental compliance and safety measures, which can significantly affect operational expenses.
Factors Affecting Price
Variability in raw material costs, energy prices, and regulatory compliance expenses are primary factors that affect cyanide pricing. Market demand, particularly from the gold mining industry, also plays a crucial role.
Availability in Global Markets
Sodium cyanide is more widely available in global markets due to its extensive use in gold mining. Potassium cyanide, while available, sees more limited use and is typically sourced for specific industrial applications. The distribution and supply chain logistics also impact availability.
Supply Chain Considerations
Impact of Geopolitical Factors
Geopolitical tensions and trade policies can affect the supply chain of cyanides, influencing availability and cost. Export restrictions or tariffs imposed by cyanide-producing countries can disrupt supply to global markets.
Transportation and Storage Costs
Transporting and storing cyanides safely involves specialized containers and regulatory compliance, contributing to higher costs. The hazardous nature of these chemicals necessitates strict security measures during transit, further increasing expenses.
Regulatory and Legal Aspects
International Regulations
Overview of Global Safety Standards
The International Cyanide Management Code (ICMC) is a voluntary program aimed at reducing the environmental and health risks of cyanide used in gold mining. Compliance with the ICMC or equivalent national regulations is essential for companies involved in the production, transport, and use of cyanide.
Differences in Regulatory Classifications
Regulatory classifications of sodium and potassium cyanide vary between countries, but both are generally classified as hazardous substances. The specific storage, handling, and disposal requirements can differ, reflecting the local regulatory environment.
Legal Restrictions
Use Restrictions in Various Industries
Legal restrictions on the use of cyanides aim to protect public health and the environment. These restrictions often pertain to concentration limits, application methods, and disposal practices in industries such as mining and chemical manufacturing.
Implications for Manufacturers and End-Users
Manufacturers and end-users of cyanides must navigate a complex landscape of local and international regulations. Compliance ensures not only legal operation but also the safety of workers and communities. Violations can result in fines, operational shutdowns, and reputational damage.
Frequently Asked Questions
What is the main use of sodium cyanide and potassium cyanide?
Sodium cyanide is predominantly used in the mining industry to extract gold and silver from ores through a process called cyanidation. Potassium cyanide, on the other hand, has its primary application in organic synthesis, where it serves as a precursor or reagent in the production of various organic compounds. It is also employed in electroplating and as a photographic fixer.
Why is sodium cyanide preferred over potassium cyanide in gold mining?
Sodium cyanide is preferred in gold mining due to its exceptional solubility in water, which enhances the efficiency of the gold leaching process. Its cost-effectiveness and availability also contribute to its preference over potassium cyanide in the extraction of precious metals.
How are sodium cyanide and potassium cyanide disposed of safely?
Safe disposal of sodium and potassium cyanide involves neutralizing their toxic effects through oxidation or hydrolysis processes that convert cyanide into less harmful substances like cyanates and eventually to carbon dioxide and nitrogen. These processes require strict adherence to environmental protection standards to prevent harm to wildlife and aquatic ecosystems.
Conclusion
Sodium cyanide and potassium cyanide, while chemically similar, exhibit distinct differences that render them suitable for various applications across industries. Their roles, from metal extraction to organic synthesis, underscore the importance of these compounds despite their inherent risks. Proper understanding and handling of these chemicals are paramount to leveraging their benefits while minimizing environmental and health impacts.
The discussion on sodium and potassium cyanide illuminates the complexity of these substances beyond their toxicity. It reveals the nuanced considerations necessary for their industrial application, emphasizing the balance between utility and safety. As industries continue to rely on these chemicals, ongoing research and regulation will be essential in ensuring their use remains both effective and responsible.