Sodium aluminate and sodium meta aluminate are two compounds that have a lot in common, yet have some distinct differences. In this blog post, we’ll look at the differences between the two compounds, including their chemical structure, physical properties, and industrial uses.
With this understanding, you’ll have a better understanding of the two compounds and how they can be used in various applications.
Chemical composition of sodium aluminate and sodium meta aluminate
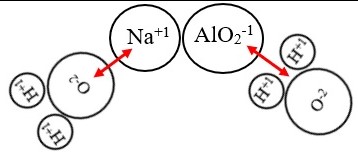
The chemical composition of sodium aluminate and sodium meta aluminate is quite similar, yet there are some differences that should be noted. Sodium aluminate is a white, odorless salt composed of sodium, aluminum, and oxygen. Sodium meta aluminate is composed of sodium, aluminum, and two oxygen atoms.
Sodium meta aluminate is composed of sodium, aluminum, and two oxygen atoms. Both are water-soluble salts used in various industrial and commercial applications. The main difference between sodium aluminate and sodium meta aluminate is the number of oxygen atoms present.
While sodium aluminate has one oxygen atom, sodium meta aluminate has two. This difference in oxygen content affects the chemical properties of each compound, such as solubility, pH, and reactivity.
Additionally, sodium aluminate is more reactive than sodium meta aluminate. Overall, sodium aluminate and sodium meta aluminate are both useful in certain industrial and commercial applications, but the difference in their chemical compositions should be taken into consideration when choosing the right compound for the job.
Differences in properties between sodium aluminate and sodium meta aluminate
Sodium Aluminate and Sodium Meta Aluminate are two chemical compounds that are very similar in form, but have some distinct differences in their properties. Sodium Aluminate is a white, odorless powder that has a strong alkaline taste, while Sodium Meta Aluminate is a yellowish-brown powder that has a mild alkaline taste. Sodium Aluminate is insoluble in water, while Sodium Meta Aluminate is slightly soluble.
Additionally, Sodium Aluminate has a high pH of 12, while Sodium Meta Aluminate has a much lower pH of
The most significant difference between the two is that Sodium Aluminate is used as a water softener, while Sodium Meta Aluminate is used as a corrosion inhibitor.
Applications of sodium aluminate and sodium meta aluminate
Sodium aluminate and sodium meta aluminate both have many applications in a variety of industries. However, it is important to understand the differences between the two.
Sodium aluminate is an anionic compound composed of sodium and alumina, while sodium meta aluminate is a neutral compound composed of sodium, alumina, and hydroxide. Both are used in many industries, but there are some key differences. Sodium aluminate is used as a chelating agent, while sodium meta aluminate is used to produce aluminum.
Additionally, sodium aluminate is used in water treatment applications, while sodium meta aluminate is used in the production of ceramics. Understanding the differences between these two compounds is essential in order to make the best use of them for various applications.
Benefits and drawbacks of sodium aluminate and sodium meta aluminate

Sodium aluminate and sodium meta aluminate are both chemical compounds that contain sodium and aluminum. Although they share some similarities, there are also some important differences between them. In terms of their benefits, sodium aluminate is often used in water treatment to reduce the pH and remove metals from the water, while sodium meta aluminate is used as a flame retardant and corrosion inhibitor.
However, both compounds can also have drawbacks. Sodium aluminate can be toxic if ingested, and sodium meta aluminate can be corrosive to some metals.
It is important to consider these factors when choosing which compound to use. Ultimately, the difference between sodium aluminate and sodium meta aluminate comes down to their properties and uses, so it is important to research and understand their advantages and disadvantages before deciding which one to use.
Considerations before choosing between sodium aluminate and sodium meta aluminate
When choosing between sodium aluminate and sodium meta aluminate, it’s important to consider the differences between the two. Sodium aluminate is an inorganic compound composed of sodium and alumina, while sodium meta aluminate is an inorganic compound composed of sodium, alumina, and metasilicate.
The most notable difference between the two is their reactivity. Sodium aluminate is more reactive than sodium meta aluminate, making it a better choice for applications that require greater reactivity. Sodium aluminate is also more soluble in water than sodium meta aluminate, making it a better choice for applications that require greater water solubility.
Finally, sodium aluminate is more easily dissolved in alkaline solutions than sodium meta aluminate, making it a better choice for applications that require greater alkalinity. Ultimately, the best choice between sodium aluminate and sodium meta aluminate will depend on the application at hand.
Conclusion
In conclusion, sodium aluminate and sodium meta aluminate are both compounds of sodium, but they differ in chemical composition. Sodium aluminate contains sodium, aluminum, and oxygen in a ratio of 1:2:3, while sodium meta aluminate contains sodium, aluminum, and oxygen in a ratio of 1:1:
Sodium aluminate is used in water treatment and industrial production, while sodium meta aluminate is used in the production of detergents and other chemicals. Both compounds have their own unique properties and applications, so it is important to understand the difference between them when choosing which compound to use.

