Statistical measures play a critical role in interpreting research findings, especially in the health sciences. Relative risk and odds ratio stand out as two key metrics used to assess and communicate the relationship between exposures and outcomes in medical and public health research. Their correct application and interpretation can profoundly influence decisions in healthcare policy, clinical practice, and patient counseling.
Relative risk compares the probability of an event occurring in two different groups, offering a direct measure of risk increase or decrease associated with a particular exposure. In contrast, the odds ratio compares the odds of an event occurring in one group to the odds in another, providing an indirect measure of risk that is particularly useful in case-control studies where actual risk cannot be directly measured.
Understanding the difference between these two measures is essential for accurately interpreting study results. Relative risk gives a more intuitive measure of the actual risk, while odds ratio can estimate the relative risk in studies where direct calculation of risk is not possible. This distinction helps researchers and clinicians to choose the appropriate statistic based on the design and objectives of their study.
Basics of Risk
Definition of Risk
In health research and clinical studies, risk refers to the probability or chance that an event will occur within a specific period or in a specific situation. This event can be the development of a disease, the occurrence of a side effect, or any other health outcome. Risk is a foundational concept in epidemiology and healthcare decision-making, as it helps to quantify the likelihood of positive or negative outcomes.
How Risk is Used in Studies
Risk is utilized in studies to:
- Assess the impact of certain factors, such as lifestyle or genetic predisposition, on health outcomes.
- Compare the safety and efficacy of treatments or interventions.
- Guide clinical decision-making and policy formulation, prioritizing interventions that reduce risk.
Relative Risk
Definition and Explanation
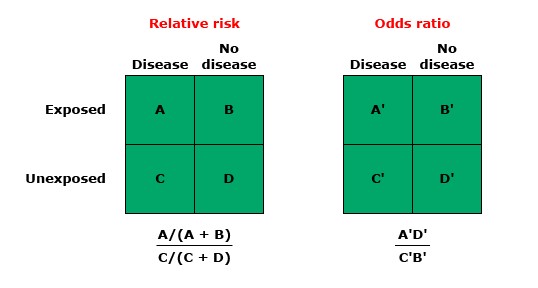
Relative Risk (RR), also known as the risk ratio, measures the ratio of the probability of an event occurring in the exposed group versus a non-exposed group. It’s a direct way to compare the risk in two different groups and is particularly useful in prospective studies, such as cohort studies.
Calculating Relative Risk
The calculation involves a few straightforward steps:
- Identify the number of cases (events) in both the exposed and non-exposed groups.
- Calculate the risk in each group by dividing the number of cases by the total number of individuals in that group.
- Divide the risk in the exposed group by the risk in the non-exposed group to find the RR.
Interpretation and Examples
- RR = 1 indicates no difference in risk between the two groups.
- RR > 1 suggests an increased risk in the exposed group.
- RR < 1 implies a decreased risk in the exposed group.
For instance, if a study finds that smokers have a RR of 2 for developing lung cancer compared to non-smokers, it means smokers are twice as likely to develop lung cancer.
Odds Ratio
Definition and Explanation
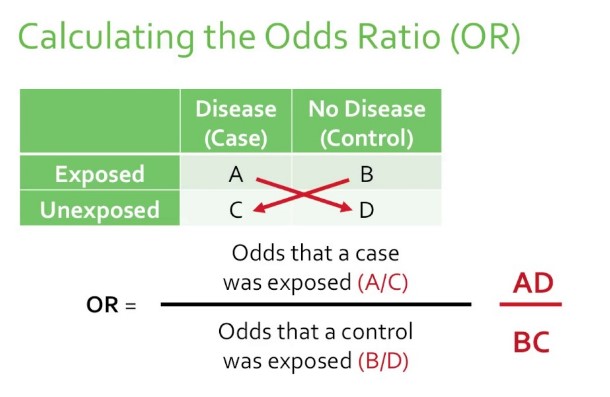
Odds Ratio (OR) compares the odds of an event occurring in one group to the odds of it occurring in another group. It’s often used in case-control studies where the direct calculation of risk is not possible. The OR provides an estimate of the relative risk in these scenarios.
Calculating Odds Ratio
To calculate the OR:
- Calculate the odds of the event in both groups (the ratio of cases to non-cases).
- Divide the odds in the exposed group by the odds in the non-exposed group.
Interpretation and Examples
- OR = 1 means there is no difference in odds between the two groups.
- OR > 1 indicates increased odds of the event occurring in the exposed group.
- OR < 1 suggests decreased odds in the exposed group.
For example, if a study shows an OR of 3 for the association between a diet high in processed foods and the development of cardiovascular disease, it indicates that those who eat a high processed food diet have three times the odds of developing cardiovascular disease compared to those who don’t.
Comparative Analysis
Differences in Definition
While Relative Risk directly measures the increase or decrease in risk, the Odds Ratio estimates the odds of an event happening, providing an indirect measure of risk.
When to Use Each Measure
- Use RR in prospective studies where you can directly observe and compare the risk between groups.
- Use OR in retrospective or case-control studies where the direct calculation of risk is not feasible.
Advantages and Limitations
RR is more intuitive and directly reflects the risk but can only be used when the incidence of the outcome is known. OR, on the other hand, can be applied in a wider range of study designs but may overestimate risk, especially in common outcomes.
Applications in Research
Epidemiological Studies
Both measures are crucial in epidemiological studies to understand the relationship between risk factors and health outcomes, guiding public health interventions.
Clinical Trials
They are used to compare the effectiveness and safety of new treatments or interventions against standard or no treatment.
Decision Making in Healthcare
Understanding RR and OR aids healthcare providers in making informed decisions about patient care and treatment options, enhancing patient outcomes and safety.
Misinterpretation Risks
Common Misunderstandings
Misinterpretations of Relative Risk (RR) and Odds Ratio (OR) often arise from a lack of understanding of their definitions and proper use. One common mistake is confusing OR for RR, especially in the interpretation of results. Since OR can overestimate the risk, especially when the outcome is common, equating it with RR may lead to exaggerated perceptions of the association between exposure and outcome. Another misunderstanding involves ignoring the study design, which dictates the appropriate measure to use, potentially leading to incorrect conclusions.
Impact on Research Outcomes
Misinterpretations can significantly impact research outcomes, including:
- Misguided public health recommendations, where the perceived risk might lead to unnecessary alarm or complacency.
- Incorrect clinical guidelines that could affect treatment decisions and patient care.
- Flawed policy-making due to an over- or underestimation of health risks.
Case Studies
Real-world Examples of Relative Risk
A classic example involves the link between smoking and lung cancer. A landmark study found that smokers had a RR of about 15 for developing lung cancer compared to non-smokers. This clear, direct measure of risk helped solidify the causal relationship between smoking and lung cancer, leading to public health campaigns and policy changes.
Real-world Examples of Odds Ratio
An example of OR usage can be found in studies examining the relationship between aspirin use and heart attack prevention. In a case-control study, researchers might find that the odds of regular aspirin users experiencing a heart attack are significantly lower compared to non-users, with an OR less than 1. This suggests a protective effect of aspirin against heart attacks, informing clinical advice on preventive measures.
Best Practices
Guidelines for Using Relative Risk and Odds Ratio
To ensure accuracy and avoid misinterpretation, follow these guidelines:
- Select the appropriate measure based on your study design. Use RR for prospective studies and OR for case-control studies.
- Consider the incidence of the outcome. If the outcome is common, be cautious in interpreting OR as it may overestimate risk.
- Contextualize your findings. Always discuss the results in the context of the existing literature and potential biases in your study.
Tips for Accurate Interpretation
To accurately interpret RR and OR, consider the following tips:
- Assess the confidence intervals (CI). A CI that includes 1 for RR or OR suggests that the association may not be statistically significant.
- Understand the baseline risk. The impact of an exposure is more meaningful when considered in the context of the baseline or background risk of the population.
- Look at absolute risk. Alongside RR and OR, consider the absolute risk difference to understand the practical significance of the findings.
- Use plain language when communicating results to ensure that they are accessible to non-specialists, including patients and policymakers.
Frequently Asked Questions
What is Relative Risk?
Relative risk is a statistic that measures the likelihood of an event occurring in one group compared to its occurrence in another group. It is often used in prospective studies, like cohort studies, to assess the effect of certain exposures on the development of outcomes.
How is Odds Ratio Different from Relative Risk?
The odds ratio compares the odds of an event occurring in one group to the odds of it occurring in another group. Unlike relative risk, which directly measures risk, the odds ratio provides an indirect measure that is particularly useful in retrospective studies, such as case-control studies.
Why is Understanding These Measures Important?
Understanding relative risk and odds ratio is crucial for correctly interpreting the results of medical research. These measures help in determining the strength of the association between exposure and outcomes, guiding clinical decision-making, and influencing public health policies.
Can Odds Ratio Overestimate Risk?
Yes, the odds ratio can overestimate the risk, especially in studies where the event of interest is common. This overestimation occurs because the odds ratio can significantly diverge from the actual risk, making it critical to interpret with caution, particularly in high-incidence settings.
Conclusion
The distinctions between relative risk and odds ratio are not merely academic; they have practical implications for how study findings are applied in clinical practice and public health. A clear understanding of these measures allows healthcare professionals and researchers to make informed decisions, accurately gauge the impact of exposures on health outcomes, and communicate risks effectively to patients and the public.
Emphasizing the appropriate use and interpretation of these statistics is essential. By grasping the nuances of relative risk and odds ratio, we can ensure that health research continues to provide valuable insights that are both accurate and meaningful, guiding evidence-based practices that improve patient outcomes and public health.