Heterocyclic compounds, which feature rings that contain atoms of at least two different elements, are pivotal in the field of chemical science. Among these, quinoline and isoquinoline are two prominent examples that have been extensively studied due to their unique structures and diverse applications. These compounds are not only fundamental in organic chemistry but also play crucial roles in various industrial processes.
Quinoline and isoquinoline are aromatic heterocyclic compounds, structurally similar yet distinctly different. Quinoline is a fused ring compound, combining a benzene ring with a pyridine ring. In contrast, isoquinoline also merges these two components but arranges them in an alternative configuration. This subtle rearrangement leads to significant differences in their chemical behavior and applications.
Understanding the differences between these compounds enriches our knowledge of chemical reactions and their practical implications in the pharmaceutical industry, among others. This insight is essential for chemists, researchers, and industries that rely on precise chemical properties and reactions to innovate and create effective products.
Quinoline Basics
Chemical Structure
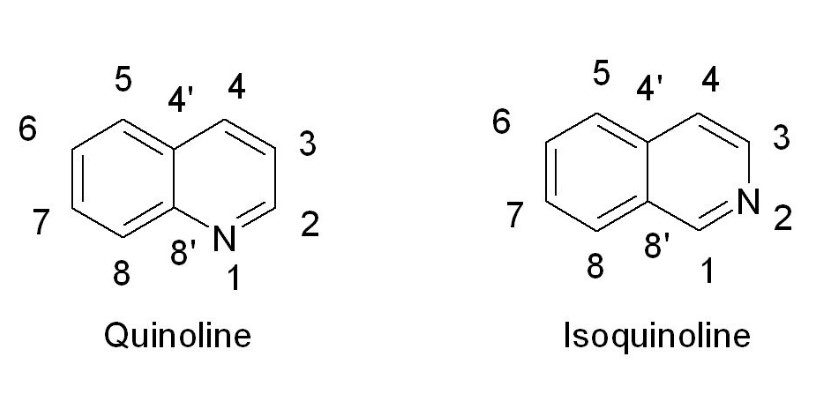
Quinoline is a heterocyclic aromatic compound, featuring a unique blend of a benzene ring fused to a pyridine ring. This structure is often depicted as having a six-membered benzene ring joined to a nitrogen-containing nine-membered pyridine ring. The nitrogen atom is positioned at the second carbon in the six-member ring, a key factor in its chemical behavior.
Properties and Characteristics
Quinoline is known for its bitter taste and strong, unpleasant odor, resembling that of fish. It is a colorless to pale yellow liquid at room temperature, exhibiting moderate solubility in water and high solubility in organic solvents. This compound demonstrates notable stability, resisting decomposition in neutral or slightly acidic environments.
- Boiling Point: Around 238 degrees Celsius
- Melting Point: -15 degrees Celsius
- Density: 1.09 g/cm³
These properties make quinoline a valuable solvent and a reagent in various chemical reactions, particularly in the synthesis of other complex organic compounds.
Production Methods
Quinoline is predominantly produced through the Skraup synthesis, a traditional method involving the reaction of aniline, glycerol, sulfuric acid, and nitrobenzene. This process, while effective, requires careful handling due to the use of high temperatures and corrosive materials. Alternative methods include:
- Friedländer synthesis: Combining o-aminoaryl ketones with aldehydes under acidic conditions.
- Catalytic methods: Employing catalysts to facilitate the reaction of aniline with aldehydes, leading to more efficient quinoline production with potentially lower environmental impact.
Isoquinoline Essentials
Chemical Structure
Isoquinoline, similar to quinoline, includes a benzene ring fused with a pyridine ring. However, in isoquinoline, the nitrogen atom occupies the first position of the six-member ring, adjacent to the junction with the benzene ring. This small shift significantly alters the compound’s chemical properties.
Properties and Characteristics
Isoquinoline is slightly less soluble in water compared to quinoline and is also a colorless to pale yellow liquid. It shares the characteristic strong odor but tends to be less volatile:
- Boiling Point: 243 degrees Celsius
- Melting Point: 23 degrees Celsius
- Density: 1.10 g/cm³
These features make isoquinoline useful in more specialized chemical applications, particularly in the synthesis of natural products and pharmaceuticals.
Synthesis Techniques
Isoquinoline is typically synthesized using the Pictet-Spengler reaction, where a β-phenylethylamine is treated with an aldehyde or ketone in acidic conditions. This method is particularly favored for its precision in ring closure, which is crucial for creating specific isoquinoline derivatives.
Structural Differences
Core Structure Analysis
At first glance, quinoline and isoquinoline may seem nearly identical, but a closer examination reveals critical differences in their structural orientation. The placement of the nitrogen atom influences not only physical properties but also reactivity.
Key Molecular Variations
The molecular structure influences key characteristics such as electronic distribution and reactivity at different sites of the molecule. For instance, the electron density around the nitrogen atom in isoquinoline is slightly higher than in quinoline, affecting its nucleophilic and electrophilic properties.
Visual Diagrams Comparison
- Quinoline: The nitrogen is at the 2-position.
- Isoquinoline: The nitrogen is at the 1-position.
These diagrams help visualize how a slight change in structure can lead to notable differences in chemical behavior.
Chemical Properties
Reactivity with Common Agents
Both compounds react with electrophilic and nucleophilic agents, but isoquinoline’s increased electron density at the nitrogen atom makes it more reactive toward electrophiles.
Stability Factors
Isoquinoline shows slightly greater thermal stability compared to quinoline, attributed to the positioning of the nitrogen which influences the distribution of pi-electrons across the molecule.
Solubility and Boiling Points
While both compounds have similar solubility in organic solvents, the slight differences in boiling points can be pivotal in their application, particularly in distillation and purification processes.
Applications in Industry
Pharmaceutical Uses
Quinoline and isoquinoline are pivotal in pharmaceutical synthesis, with their derivatives forming the backbone of several vital medications. Quinoline is instrumental in creating antimalarial drugs such as chloroquine and mefloquine, crucial for treating and controlling malaria in endemic regions. Isoquinoline derivatives are used in making vasodilators, antispasmodics, and analgesics, enhancing their significance in medical treatments.
- Antimalarial Drugs: Chloroquine synthesis involves several quinoline-based intermediates.
- Pain Management: Isoquinoline compounds are used to synthesize papaverine, a non-narcotic analgesic.
Dyes and Pigments
Both compounds find extensive use in the production of dyes and pigments. Quinoline dyes are known for their vibrant colors and are used in textiles, inks, and food colorants. Isoquinoline derivatives serve as intermediates in the synthesis of yellow and orange dyes, widely used in industries requiring high color fastness.
Other Commercial Uses
Beyond pharmaceuticals and dyes, these heterocycles contribute to various sectors including agriculture, where they are used in pesticides and herbicides, and in the production of rubber chemicals and corrosion inhibitors, showcasing their versatility.
Biological Activities
Medicinal Implications
The biological activity of quinoline and isoquinoline extends into significant medicinal properties. They are studied for their potential in treating neurodegenerative diseases and cancer, with ongoing research into their efficacy and mechanisms.
Toxicological Aspects
Despite their benefits, quinoline and isoquinoline pose certain health risks. They are identified as potentially harmful if ingested, inhaled, or absorbed through the skin, necessitating careful handling and usage guidelines in industrial and laboratory settings.
Recent Research Findings
Recent studies have highlighted the potential of isoquinoline derivatives in inhibiting cancer cell growth, suggesting a promising avenue for new cancer treatments. This area of research is rapidly evolving, with numerous studies aimed at enhancing the therapeutic index of these compounds.
Analytical Methods
Spectroscopy Techniques
- Nuclear Magnetic Resonance (NMR): Used to determine the structure and electronic environment of quinoline and isoquinoline.
- Infrared (IR) Spectroscopy: Helps in identifying functional groups, aiding in the analysis of various derivatives.
Chromatography Approaches
Chromatography, particularly High-Performance Liquid Chromatography (HPLC), plays a crucial role in purifying and quantifying quinoline and isoquinoline derivatives, ensuring purity and concentration are within usable limits for pharmaceutical applications.
Laboratory Protocols
Effective laboratory protocols are established for the synthesis and handling of quinoline and isoquinoline, focusing on safety, yield optimization, and environmental impact. These protocols are essential for researchers and industry professionals to follow to maintain integrity and safety in their work.
Environmental Impact
Ecological Risks
Quinoline and isoquinoline can pose significant ecological risks if not managed properly. Their potential for bioaccumulation and toxicity to aquatic life necessitates stringent waste management and disposal practices.
Disposal and Degradation
Proper disposal methods are critical in minimizing environmental impact. Techniques such as incineration and biodegradation are employed, but they must be carefully managed to avoid releasing harmful byproducts into the environment.
Regulatory Guidelines
Regulatory bodies have set guidelines for the safe handling, usage, and disposal of quinoline and isoquinoline. These regulations help ensure that their use in industries does not pose a significant threat to human health and the environment, balancing industrial benefits with ecological safety.
FAQs
What is Quinoline?
Quinoline is an aromatic nitrogen-containing heterocycle used primarily as a solvent and chemical building block. It’s widely utilized in the synthesis of various pharmaceuticals, including antimalarial drugs, antifungals, and antibiotics.
How is Isoquinoline different from Quinoline?
Isoquinoline, like quinoline, is a nitrogen-containing aromatic heterocycle. However, its nitrogen atom is located in a different position within the ring structure, which significantly affects its chemical properties and uses, particularly in the synthesis of analgesics and antitumor agents.
Where are Quinoline and Isoquinoline found?
Both compounds are found in coal tar, crude oil, and as byproducts in various industrial processes. Additionally, they occur naturally in several plants and are synthetically produced for commercial use.
Why are these compounds important?
Quinoline and isoquinoline are crucial due to their extensive use in medicinal chemistry. They serve as key intermediates in the manufacture of various drugs and have notable roles in research involving synthetic and medicinal organic chemistry.
Conclusion
In exploring quinoline and isoquinoline, we uncover the intricate balance of structure and function inherent to chemical compounds. Their unique configurations not only differentiate their chemical properties but also define their roles across various industries. The study of these differences is not just academic but a practical necessity, driving innovations in pharmaceuticals and beyond.
As we advance our understanding of these compounds, we pave the way for future scientific breakthroughs and the development of new technologies. This continuous exploration not only deepens our comprehension of chemistry but also enhances our ability to solve complex problems in medicine and environmental science.