Crystals are not just aesthetically pleasing objects; they are also fundamental to our understanding of material science. At the microscopic level, the arrangement of atoms within a crystal defines its entire set of properties, from strength to conductivity. Among these, the hexagonal structures in crystals represent a crucial area of study due to their unique properties and applications.
The difference between a primitive hexagonal unit cell and hexagonal closed packing lies in their atomic arrangements and spatial efficiency. A primitive hexagonal unit cell is simpler, consisting of atoms at each corner of a hexagon and one at the center of the hexagon, making it less densely packed. In contrast, hexagonal closed packing is a more complex and tightly packed structure with layers of atoms arranged in a sequence that maximizes spatial utilization and structural stability.
Hexagonal structures are pervasive in various materials and influence their mechanical strength, density, and other physical properties. The way atoms are packed can affect everything from the durability of a metal to the efficiency of a semiconductor. Understanding these differences not only helps in material selection but also in designing new materials with desired properties.

Hexagonal Unit Cells
Basic Concepts
In the realm of crystallography, a unit cell is the smallest repeating structure of a crystal lattice that retains the overall symmetry of the crystal. The hexagonal unit cell, a pivotal structure in materials science, is distinguished by its unique six-sided symmetry. This symmetry is not merely aesthetic; it impacts the cell’s physical properties and its interaction with other cells in a lattice.
A hexagonal lattice is comprised of parallel rows of atoms, each offset to nest in the gaps of adjacent rows. This configuration can be visualized as rows of circles in a staggered pattern, where each circle represents an atom. Such an arrangement is pivotal in materials that exhibit anisotropic properties, meaning their properties vary with direction along the crystal.
Structure and Parameters
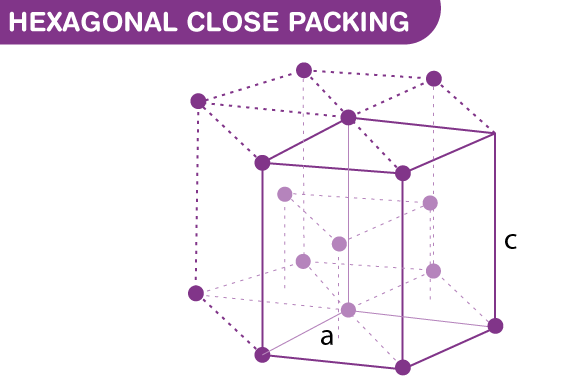
The structure of a hexagonal unit cell is defined by its lattice parameters: the cell dimensions along its axes. Specifically, a hexagonal unit cell has two parameters:
- a (the cell’s width): identical for all six sides of the hexagon,
- c (the cell’s height): perpendicular to the hexagonal face.
These parameters are crucial as they determine the density and packing efficiency of the cell in the crystal lattice. A typical feature of the hexagonal structure is the c/a ratio, an essential factor in determining the mechanical and thermal properties of the material.
Hexagonal Closed Packing
Definition and Features
Hexagonal closed packing (HCP) is a type of atomic arrangement that offers close packing of atoms with high efficiency and stability. This structure is characterized by its sequence of layered atoms, each layer offset from the one below it, allowing for a denser packing compared to simpler structures like the primitive hexagonal unit cell.
Key features of HCP include:
- High packing density: achieves a packing efficiency of about 74%, which is among the highest for crystalline structures.
- Directional properties: due to its unique layered arrangement, HCP materials can exhibit different properties in different directions (anisotropy).
Arrangement of Atoms
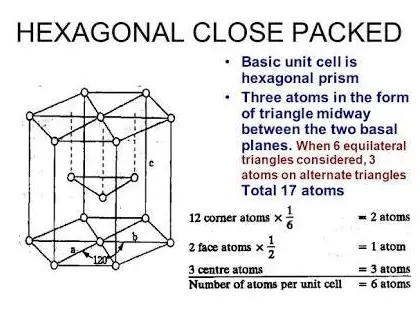
In the hexagonal closed packing structure, atoms are arranged in layers, with each atom touching twelve others. This arrangement can be divided into two alternating types of layers, often labeled as A and B. In an A layer, atoms are aligned at the vertices of equilateral triangles. In a B layer, atoms nest in the triangular gaps left by the A layer above it.
This AB sequence repeats throughout the material, which not only maximizes spatial utilization but also enhances the stability of the crystal by distributing atomic bonds uniformly across the structure.
Comparing Structures
Geometric Differences
While both the primitive hexagonal unit cell and hexagonal closed packing are built on a hexagonal geometry, their structural differences are significant:
- Unit cell simplicity vs. packing complexity: The primitive cell has a simpler, less dense arrangement compared to the complex, tightly packed HCP.
- Volume and space efficiency: HCP utilizes space more efficiently due to its layered, close-packed structure.
Atomic Coordination
The arrangement of atoms within these structures also varies markedly in terms of atomic coordination:
- Primitive hexagonal unit cells have atoms at each corner of a hexagon with possibly an additional atom at the center, each atom coordinating with fewer neighbors compared to HCP.
- Hexagonal closed packing structures ensure each atom is in direct contact with twelve others, enhancing structural cohesion and stability.
Volume and Density
Calculating Unit Cell Volume
The volume of a unit cell is a key factor in determining the physical properties of a crystal. For hexagonal unit cells, the volume can be calculated using the formula:
𝑉=332𝑎2𝑐V=233a2c
where 𝑎a is the cell edge length, and 𝑐c is the height of the cell. This calculation reflects the space each cell occupies within the lattice, providing insights into the material’s structure.
Density Comparisons
Density is another critical property influenced by the unit cell’s volume and the atomic mass present within the cell. The density 𝜌ρ of a crystal is computed as follows:
𝜌=𝑍×𝑀𝑉×𝑁𝐴ρ=V×NAZ×M
where:
- 𝑍Z = number of formula units per unit cell,
- 𝑀M = molar mass of the compound,
- 𝑉V = volume of the unit cell,
- 𝑁𝐴NA = Avogadro’s number.
Comparing densities of materials with primitive hexagonal cells and those with hexagonal closed packing can highlight differences in their packing efficiencies and structural compactness, crucial for applications requiring high density and strength.
Impact on Material Properties
Mechanical Properties
The arrangement of atoms within a crystal directly influences its mechanical properties, such as hardness, elasticity, and tensile strength. Hexagonal structures, due to their unique lattice arrangements, often show distinct directional mechanical properties. For instance, materials with hexagonal closed packing typically exhibit high resistance to deformation and excellent ductility along certain crystallographic directions due to the tight atomic packing.
Thermal and Electrical Conductivity
Thermal conductivity in hexagonal structures can vary significantly depending on the packing of the unit cells. Generally, more densely packed structures allow for better heat transfer across the material. Similarly, electrical conductivity is affected by the arrangement of atoms and the presence of free electrons. Materials with hexagonal closed packing, like magnesium, often have better conductivity properties due to the minimal disruption in electron movement through the dense lattice.
Applications in Industry
Aerospace and Automotive
In the aerospace and automotive industries, materials need to withstand extreme conditions while maintaining a low weight. Materials with hexagonal structures, such as titanium alloys, are prized for their high strength-to-weight ratio and excellent high-temperature performance. These materials are fundamental in designing components that are both lightweight and capable of performing under high stress and temperature conditions.
Electronics and Ceramics
In the electronics industry, materials with hexagonal structures, such as zinc oxide, play a crucial role in developing semiconductors, varistors, and transducers. The hexagonal lattice allows for unique electrical properties, which are essential for piezoelectric sensors and other electronic components.
Ceramics, too, benefit from hexagonal structures, especially in terms of thermal stability and wear resistance. For example, silicon carbide, used in high-performance ceramic applications, adopts a hexagonal crystal structure that provides exceptional thermal conductivity and hardness, ideal for high-stress environments.
Frequently Asked Questions
What is a Primitive Hexagonal Unit Cell?
A primitive hexagonal unit cell features atoms at the vertices of a hexagon and a single atom at the center. This arrangement forms the simplest repeating unit in some crystalline structures, showing how atoms bond and repeat in specific directions.
How is Hexagonal Closed Packing Arranged?
In hexagonal closed packing, atoms are tightly packed in alternating layers, where each atom is surrounded by 12 others. This arrangement leads to a highly dense structure, often found in metals like cobalt and zinc, enhancing their structural cohesion.
Why is Hexagonal Packing Important?
Hexagonal packing influences the physical properties of materials, including their density, melting points, and strength. Understanding these packings helps scientists and engineers tailor materials for specific applications, from aerospace engineering to electronics.
What Materials Use Hexagonal Structures?
Many metals and alloys, such as titanium, magnesium, and some stainless steels, adopt hexagonal structures. These materials are chosen for their strength-to-weight ratio and resistance to high temperatures and corrosion, making them ideal for challenging environments.
Conclusion
The exploration of primitive hexagonal unit cells and hexagonal closed packing reveals significant insights into material science. By comparing these two structures, we can better understand their implications on material properties and how they can be manipulated to meet specific engineering requirements.
The knowledge of hexagonal structures not only supports the advancement of existing materials but also paves the way for innovations in material science. As technology evolves, so too does our ability to engineer materials at an atomic level, enhancing their capabilities and applications in various industries.