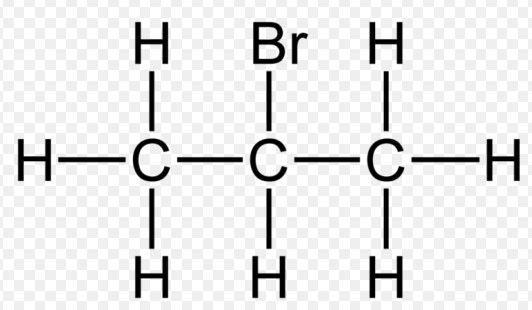
Halogenoalkanes, also known as alkyl halides, are a class of organic compounds where one or more halogen atoms are bonded to an alkane’s carbon atoms. They play a pivotal role in both the academic study of chemistry and the practical applications of chemical reactions, ranging from pharmaceuticals to agricultural chemicals. Their diversity and reactivity make them essential subjects of study in organic chemistry.
The difference between primary, secondary, and tertiary halogenoalkanes lies in the positioning of the halogen atom relative to the carbon chain. Primary halogenoalkanes have the halogen atom attached to a carbon that is bonded to only one other carbon. Secondary halogenoalkanes have the halogen atom on a carbon bonded to two other carbons, while tertiary halogenoalkanes are those in which the halogenated carbon is bonded to three other carbons.
These variations influence the physical and chemical properties of the halogenoalkanes, including their reactivity, boiling points, and solubility. Understanding these differences is crucial for chemists to predict reactions, synthesize new compounds, and develop applications ranging from solvents to anesthetics. The classification into primary, secondary, and tertiary forms provides a framework for discussing their unique characteristics and applications.
Halogenoalkanes Explained
What Are Halogenoalkanes
Halogenoalkanes, often referred to as alkyl halides, are a significant group of organic compounds characterized by the presence of one or more halogen atoms (such as fluorine, chlorine, bromine, or iodine) attached to the carbon atoms of an alkane. The inclusion of halogen atoms in these molecules introduces a variety of chemical behaviors not present in their alkane counterparts, making halogenoalkanes versatile in organic synthesis and industrial applications.
The general structure of halogenoalkanes can be simplified as R-X, where R represents an alkyl group (a chain of carbon atoms) and X denotes a halogen atom. The role of the halogen atom is pivotal; it significantly influences the compound’s physical and chemical properties, including boiling points, solubility, and reactivity. This versatility stems from the electronegativity of the halogens, which alters the electron distribution in these compounds, thereby affecting their interaction with other substances.
Classification Basis
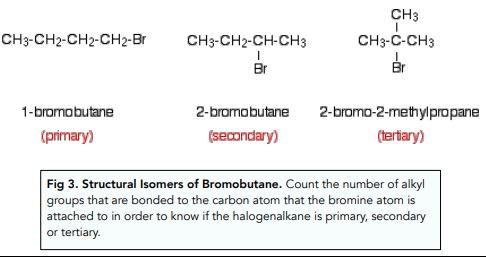
The classification of halogenoalkanes into primary, secondary, and tertiary categories is based on the carbon atom to which the halogen is bonded. This classification is crucial as it directly impacts the compound’s reactivity, a key factor in determining its role in chemical reactions and potential applications:
- Primary halogenoalkanes have the halogen atom attached to a carbon atom that is bonded to only one other carbon.
- Secondary halogenoalkanes feature the halogen atom bonded to a carbon atom connected to two other carbons.
- Tertiary halogenoalkanes are characterized by the halogen atom being attached to a carbon atom that is linked to three other carbon atoms.
Understanding this classification is fundamental in predicting the behavior of halogenoalkanes in reactions and is a primary step in the synthesis of complex organic molecules.
Primary Halogenoalkanes
Structure and Properties
Primary halogenoalkanes are characterized by their simple structure, where the halogen atom is bonded to a carbon atom that, in turn, is connected to only one other carbon. This configuration influences their physical and chemical properties significantly. Compared to secondary and tertiary halogenoalkanes, primary variants tend to have lower boiling points and are less sterically hindered, which affects their reactivity patterns, especially in nucleophilic substitution reactions.
The positioning of the halogen atom in primary halogenoalkanes makes them somewhat less reactive towards elimination reactions but more susceptible to nucleophilic attacks. This is because the carbon-halogen bond in primary halogenoalkanes is more accessible to nucleophiles, given the lower degree of steric hindrance.
Reactions and Uses
Primary halogenoalkanes undergo a range of common reactions, each showcasing their utility in both research and industrial settings. Some of these reactions include:
- Nucleophilic Substitution (SN2 reaction): This reaction is characteristic of primary halogenoalkanes due to their open structure, allowing nucleophiles easier access to the carbon-halogen bond. It results in the replacement of the halogen atom with a nucleophile.
- Hydrolysis: Primary halogenoalkanes can react with water in the presence of a base, leading to the formation of alcohols. This reaction is often utilized in the synthesis of alcohols from halogenated compounds.

Secondary Halogenoalkanes
Structure and Properties
Secondary halogenoalkanes are characterized by the halogen atom being attached to a carbon that is connected to two other carbons. This configuration results in a balance between reactivity and stability, making them distinct from both primary and tertiary halogenoalkanes. They boil at higher temperatures than their primary counterparts due to the increased molecular weight and van der Waals forces.
The reactivity of secondary halogenoalkanes is influenced by the steric hindrance and electronic effects of the two alkyl groups attached to the carbon bearing the halogen. This structure makes them moderately reactive in both nucleophilic substitution and elimination reactions. Compared to primary halogenoalkanes, the presence of an additional alkyl group provides more steric hindrance, which can slow down reactions involving large nucleophiles or bases.
Reactions and Uses
Secondary halogenoalkanes participate in a variety of chemical reactions, highlighting their versatility:
- Nucleophilic Substitution (SN1 and SN2 reactions): They can undergo both SN1 and SN2 reactions, depending on the conditions. SN2 reactions occur more slowly compared to primary halogenoalkanes due to steric hindrance. SN1 reactions are facilitated by the carbocation stability provided by the two alkyl groups.
- Elimination Reactions: Secondary halogenoalkanes are prone to elimination reactions, particularly when treated with strong bases. This reaction pathway is often exploited to create alkenes.
In practical applications, secondary halogenoalkanes are valuable in the synthesis of various organic compounds, including solvents and pharmaceuticals. Their balanced reactivity makes them ideal intermediates in organic synthesis, where precise control over reaction pathways is crucial.
Tertiary Halogenoalkanes
Structure and Properties
Tertiary halogenoalkanes feature a halogen atom attached to a carbon atom that is bonded to three other carbon atoms. This structure significantly enhances their stability towards nucleophilic substitution reactions due to the steric hindrance and the inductive effect from the three alkyl groups, making the carbon-halogen bond less accessible and the carbon center more electron-rich.
The increased steric hindrance and electron-donating effects of the alkyl groups render tertiary halogenoalkanes highly resistant to SN2 reactions. However, they are particularly reactive in SN1 reactions, where the stability of the resultant carbocation plays a critical role in the reaction mechanism.
Reactions and Uses
Tertiary halogenoalkanes are known for their unique reactions:
- Nucleophilic Substitution (SN1 reaction): They readily undergo SN1 reactions, leading to the formation of carbocations, which can then react with nucleophiles. This property is harnessed in synthesizing a wide range of compounds.
- Elimination Reactions: They are also susceptible to elimination reactions, forming alkenes. This is often used in the preparation of unsaturated compounds.
Their reactivity makes tertiary halogenoalkanes crucial in the manufacture of polymers, lubricants, and other high-molecular-weight organic compounds. Their ability to undergo specific reactions with predictability makes them valuable in industrial chemistry.
Comparative Analysis
Reactivity Patterns
The reactivity of halogenoalkanes varies significantly across the primary, secondary, and tertiary classifications. Primary halogenoalkanes are most reactive in SN2 reactions, secondary halogenoalkanes show mixed reactivity favoring both SN1 and SN2 pathways, and tertiary halogenoalkanes are predominantly reactive in SN1 reactions. The underlying factor influencing this behavior is the structure around the carbon-halogen bond, specifically how steric hindrance and carbocation stability affect the reaction mechanism.
Physical Properties
The physical properties of halogenoalkanes, such as boiling points and solubility, also vary with their structure. Generally, as the molecular weight increases from primary to tertiary, so does the boiling point due to the greater van der Waals forces. Solubility in water decreases with increasing molecular weight and branching, as the hydrophobic character of the alkyl groups becomes more pronounced.
Environmental Impact
The use of halogenoalkanes has raised environmental concerns, particularly regarding their persistence and bioaccumulation. Many halogenated compounds are resistant to degradation, leading to their accumulation in ecosystems and potential harm to wildlife and humans. The environmental impact of halogenoalkanes necessitates careful consideration in their production, use, and disposal, highlighting the need for sustainable chemistry practices and the development of less harmful alternatives.
Frequently Asked Questions
What Are Halogenoalkanes?
Halogenoalkanes, or alkyl halides, are organic compounds characterized by the presence of one or more halogen atoms (fluorine, chlorine, bromine, or iodine) attached to the carbon atoms of an alkane. These compounds are versatile in chemical reactions, serving as a fundamental building block in organic synthesis and industrial applications due to their reactivity and variability.
How Do Primary, Secondary, and Tertiary Halogenoalkanes Differ?
The classification into primary, secondary, and tertiary halogenoalkanes is based on the carbon atom to which the halogen is bonded. In primary halogenoalkanes, the halogen is attached to a carbon bonded to only one other carbon. Secondary halogenoalkanes have the halogen attached to a carbon bonded to two other carbons, while in tertiary halogenoalkanes, the halogenated carbon is bonded to three carbons. This classification affects their chemical reactivity and physical properties.
Why is the Reactivity of Halogenoalkanes Important?
The reactivity of halogenoalkanes is central to organic chemistry and industrial applications because it determines how these compounds interact in chemical reactions. Their reactivity with nucleophiles, for instance, is a cornerstone of substitution reactions, a fundamental process used to synthesize a wide array of chemicals, from medicinal drugs to agricultural products. Understanding their reactivity helps in predicting outcomes of chemical reactions and designing new compounds.
Conclusion
Halogenoalkanes, with their vast array of applications and fundamental importance in organic chemistry, embody the intricate relationship between structure and reactivity. The distinction between primary, secondary, and tertiary halogenoalkanes offers a lens through which we can appreciate the subtle nuances that govern chemical behavior. These classifications not only aid in the prediction of chemical reactions but also in the design and synthesis of new molecules, highlighting the depth and complexity of organic chemistry.
The exploration of halogenoalkanes extends beyond academic curiosity, impacting various industries and leading to innovations in pharmaceuticals, agriculture, and materials science. As we delve deeper into their study, the potential for new discoveries and applications continues to expand, underscoring the importance of understanding these compounds in all their diversity.