Light emission from matter is a fascinating phenomenon that has been studied for centuries. Photoluminescence and fluorescence are two processes by which matter emits light, but there are important differences between them. In this blog post, we’ll explore the difference between photoluminescence and fluorescence and discuss what makes them unique.
In this blog post, we’ll explore the difference between photoluminescence and fluorescence and discuss what makes them unique.
The difference between photoluminescence and fluorescence
The difference between photoluminescence and fluorescence is subtle but significant. Photoluminescence is the emission of light that is caused by the absorption of energy from a light source.
The key difference is the type of energy that is absorbed: Photoluminescence is light-based, while fluorescence is electron-based. This means that photoluminescence is more efficient at converting energy into light than fluorescence, and is often used in applications such as medical imaging.
Photoluminescence and its uses

Photoluminescence and fluorescence are two terms often used interchangeably, but they are actually quite different. Photoluminescence is the emission of light from any material when it absorbs energy, typically from ultraviolet light. Fluorescence, on the other hand, is the emission of light from a material after it has absorbed energy from light in the visible region of the spectrum.
The main difference between photoluminescence and fluorescence is the energy source used to excite the material. Photoluminescence is excited by radiation in the ultraviolet spectrum while fluorescence is excited by radiation in the visible spectrum.
In addition, the emitted light from photoluminescence is typically in the visible spectrum while the emitted light from fluorescence is usually in the ultraviolet spectrum. Technological applications for photoluminescence include light-emitting diodes (LEDs) and photovoltaic cells, while fluorescence is used in medical imaging and chemical analysis.
Fluorescence and its uses
Fluorescence is a fascinating phenomenon where light is absorbed by molecules and re-emitted at a lower energy level. While similar to photoluminescence, the two processes differ in several ways.
Fluorescence, however, involves the absorption and re-emission of light at a lower energy level. This lower-energy light is then visible to the human eye, resulting in the characteristic “glow” of many fluorescent materials.
Fluorescence has many applications in various industries, such as lighting, diagnostics, and security. In addition, fluorescence spectroscopy is an invaluable tool in the study of the properties of molecules. It can be used to study the structure, composition, and interactions of those molecules, allowing for deeper insights into the world around us.
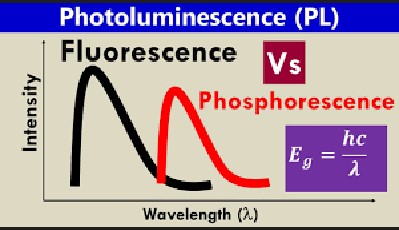
How photoluminescence and fluorescence differ in wavelengths

The physical properties of light, such as its wavelength and frequency, can be manipulated to create different effects. Photoluminescence and fluorescence are two phenomena that involve the interaction of light with a material and are responsible for a variety of phenomena in nature.
Photoluminescence typically produces light of longer wavelengths than fluorescence, resulting in a different effect. In photoluminescence, the material absorbs the energy of light, which is then re-emitted at a longer wavelength.
Fluorescence is the result of a material absorbing light of a certain wavelength and then emitting light at a shorter wavelength. This difference in the wavelengths of light produces different effects. Photoluminescence is often used in lighting technology, while fluorescence is used in medical imaging and research.
Advantages and disadvantages of photoluminescence and fluorescence

Photoluminescence and fluorescence are two different processes of light emission. While both processes involve the absorption of energy to produce light, they differ in the type of energy that is absorbed and emitted.
Photoluminescence requires the absorption of ultraviolet or visible light energy, while fluorescence requires the absorption of ultraviolet or visible light energy plus an additional energy source, such as heat or electricity. The emitted light in photoluminescence is usually of a longer wavelength than in fluorescence, and the intensity of the emitted light is usually lower. In addition, photoluminescence is a non-reversible process, meaning that once the light is emitted, it cannot be absorbed again.
Meanwhile, fluorescence is a reversible process, meaning that the light can be absorbed and re-emitted multiple times. The advantages of photoluminescence are that it is typically cheaper and simpler to manufacture than fluorescence, and it is more efficient in terms of energy usage. The main disadvantage is the lower intensity of the emitted light.
The main disadvantage is the lower intensity of the emitted light. Fluorescence has the advantage of higher intensity and the ability to be re-emitted multiple times, but the disadvantage is that it is usually more expensive and complex to produce.
Final Touch
In conclusion, the main difference between photoluminescence and fluorescence is that photoluminescence is the emission of light from a material when it is exposed to a light source, while fluorescence is the emission of light from a material when it is exposed to radiation. Photoluminescence is a spontaneous process, while fluorescence is an induced process.
Photoluminescence is generally a longer-lasting process, while fluorescence is a shorter-lasting process. Photoluminescence is used for applications such as lighting and displays, while fluorescence is used predominantly in medical imaging and chemical analysis.