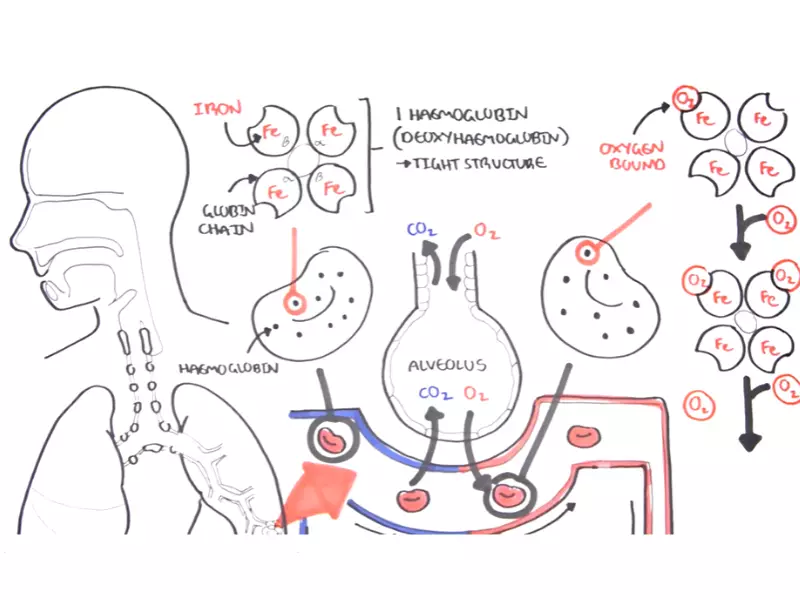
Hemoglobin, a crucial protein in red blood cells, plays a pivotal role in transporting oxygen from the lungs to the rest of the body and returning carbon dioxide back for exhalation. This protein’s function hinges on its ability to switch forms between oxygenated and deoxygenated states, a transformation vital for sustaining life. The efficiency of hemoglobin’s oxygen-carrying capacity is a cornerstone of our respiratory and circulatory systems, highlighting the protein’s significance in human physiology.
The key difference between oxygenated and deoxygenated hemoglobin lies in their oxygen binding. Oxygenated hemoglobin, formed when hemoglobin binds with oxygen in the lungs, is responsible for transporting oxygen to the body’s tissues. Conversely, deoxygenated hemoglobin, which results when oxygen is released into the tissues, carries carbon dioxide back to the lungs. This dynamic balance supports cellular respiration and energy production within the body.
Understanding the distinctions between these two forms of hemoglobin not only offers insights into fundamental physiological processes but also aids in diagnosing and managing various health conditions. The ability of hemoglobin to efficiently transport oxygen is influenced by several factors, including pH levels, temperature, and the presence of certain chemicals, which can affect its oxygen-binding capacity and, by extension, the body’s overall oxygenation status.
Hemoglobin Basics
Structure and Function
Hemoglobin, a vital protein in red blood cells, plays a crucial role in the transport of oxygen from the lungs to various parts of the body. Composed of four subunits, each containing an iron-bound heme group, hemoglobin’s structure is perfectly engineered to bind and release oxygen efficiently.
Hemoglobin Composition
Each hemoglobin molecule consists of two alpha and two beta chains, each with a heme group capable of binding one oxygen molecule. This composition allows hemoglobin to carry up to four molecules of oxygen, facilitating high oxygen transport capacity.
Role in Oxygen Transport
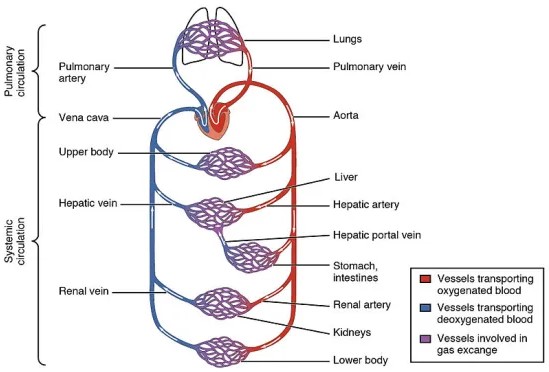
Hemoglobin transitions between oxygenated and deoxygenated states, enabling the transport of oxygen to body tissues and the return of carbon dioxide to the lungs for exhalation. This process is essential for cellular respiration, supporting energy production and metabolic processes.
Oxygenated Hemoglobin
Definition and Characteristics
Oxygenated hemoglobin, or oxyhemoglobin, forms when hemoglobin binds to oxygen in the lungs. This binding triggers a conformational change in the protein, increasing its affinity for oxygen and enabling the transport of this vital gas throughout the body.
How Oxygen Binds to Hemoglobin
Oxygen binding occurs at the heme groups of hemoglobin. The iron atom in the center of each heme group can bind one oxygen molecule, causing hemoglobin to transition to its oxygenated state. This process is facilitated by the partial pressure of oxygen in the lungs, promoting efficient oxygen loading.
Deoxygenated Hemoglobin
Definition and Differences from Oxygenated
Deoxygenated hemoglobin, or deoxyhemoglobin, is hemoglobin that has released its oxygen molecules to the body tissues. This form of hemoglobin has a lower affinity for oxygen, ready to bind to carbon dioxide and other byproducts of metabolism for transport back to the lungs.
Process of Releasing Oxygen
The release of oxygen by hemoglobin is influenced by the partial pressure of oxygen in the tissues. As oxygen is consumed during cellular respiration, its concentration decreases, prompting hemoglobin to release oxygen where it’s most needed.
Key Differences
Oxygen Binding Capacity
Oxygenated and deoxygenated hemoglobin differ significantly in their oxygen binding capacities. Oxygenated hemoglobin can bind up to four oxygen molecules, while deoxygenated hemoglobin releases these molecules to support cellular respiration.
Color Variations
The color of blood changes based on the state of hemoglobin. Oxygenated hemoglobin gives blood a bright red color, while deoxygenated hemoglobin results in a darker red to purple hue. This color change is due to the differences in the spectral properties of hemoglobin in its oxygenated and deoxygenated forms.
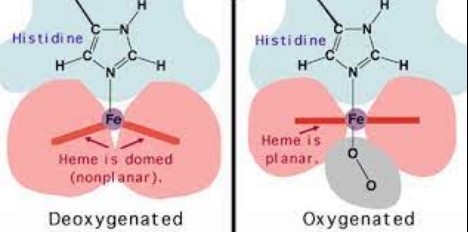
Structural Changes
Oxygenation and deoxygenation induce structural alterations in the hemoglobin molecule. Oxygen binding leads to a R state (relaxed state) conformation, which is more oxygen-affinitive. In contrast, the release of oxygen results in a T state (tense state), reducing the protein’s affinity for oxygen.
Physiological Significance
Oxygen Delivery
Hemoglobin’s ability to switch between oxygenated and deoxygenated states is key to its role in oxygen delivery. This dynamic process ensures that oxygen is efficiently distributed to tissues according to their metabolic needs.
Mechanisms of Oxygen Transport to Tissues
- Oxygen loading occurs in the lungs, where high oxygen pressure facilitates binding to hemoglobin.
- Oxygen unloading in tissues is driven by lower oxygen concentration, enabling cells to receive oxygen for metabolism.
CO2 Transport
Deoxygenated hemoglobin also plays a crucial role in carbon dioxide transport. CO2 produced by cells is bound to hemoglobin for transport back to the lungs, where it is released and exhaled.
Factors Influencing Oxygenation
Oxygenation, the process through which hemoglobin binds to oxygen in the lungs and transports it to the tissues, is influenced by several physiological and environmental factors. Understanding these factors is crucial for comprehending how the body adapts to different conditions to maintain optimal oxygen levels.
pH Levels
Bohr Effect on Oxygen Affinity
The Bohr effect is a physiological phenomenon that describes how changes in blood pH affect hemoglobin’s oxygen-binding affinity. When muscles work hard, they produce lactic acid, lowering the blood’s pH. This acidification reduces hemoglobin’s affinity for oxygen, facilitating more efficient oxygen unloading in the tissues. Conversely, in the lungs, where the pH is higher due to the expulsion of carbon dioxide, hemoglobin’s affinity for oxygen increases, enhancing oxygen uptake.
Temperature
Effects of Temperature Changes
Temperature also plays a significant role in hemoglobin’s oxygen-binding capacity. An increase in temperature, as seen in active tissues, decreases hemoglobin’s oxygen affinity, promoting oxygen release. This mechanism supports the increased metabolic demands of tissues during exercise or fever. Conversely, a lower temperature increases oxygen affinity, beneficial in the lungs for oxygen pickup.
Other Factors
2,3-DPG Levels and Altitude
2,3-Diphosphoglycerate (2,3-DPG) levels in red blood cells affect hemoglobin’s oxygen affinity. An increase in 2,3-DPG, often triggered by conditions such as high altitude, hypoxia, or anemia, decreases oxygen affinity. This adjustment facilitates oxygen delivery to tissues under low oxygen concentration conditions.
Altitude impacts oxygenation due to the reduced oxygen pressure in the atmosphere. The body compensates by increasing red blood cell production and 2,3-DPG levels, enhancing the oxygen unloading capacity of hemoglobin in tissues.
Health Implications
Hypoxia and Its Effects
Hypoxia, a condition characterized by inadequate oxygen supply to the tissues, can result from various factors, including lung diseases, heart problems, and exposure to high altitudes. Hypoxia’s effects range from short-term symptoms like fatigue and shortness of breath to severe long-term complications such as organ damage and failure.
Conditions Leading to Low Oxygen Saturation
Several conditions can lead to low oxygen saturation, including chronic obstructive pulmonary disease (COPD), heart failure, and sleep apnea. These conditions disrupt the normal oxygenation process, leading to systemic hypoxia and its associated health risks.
Diagnostic Uses
Pulse Oximetry and Blood Gas Analysis
Pulse oximetry is a non-invasive method used to measure the oxygen saturation level in the blood. It provides a quick and efficient way to monitor a patient’s oxygenation status, crucial for diagnosing and managing respiratory and cardiac conditions.
Blood gas analysis is a more comprehensive diagnostic tool that measures oxygen and carbon dioxide levels, pH, and other parameters in the blood. It offers detailed insights into a patient’s respiratory and metabolic status, aiding in the diagnosis and treatment of various conditions.
Innovations in Research
Artificial Hemoglobin
Developments and Potential Uses
Research into artificial hemoglobin seeks to create blood substitutes that can carry oxygen and carbon dioxide, offering potential solutions for blood shortages and transfusion-related issues. Recent developments have focused on encapsulating hemoglobin in polymers or creating hemoglobin-based oxygen carriers (HBOCs) that mimic natural hemoglobin’s function. These innovations hold promise for emergency medicine, surgery, and treating chronic conditions requiring frequent transfusions.
Oxygen Therapeutics
Advances in Medical Treatment
Oxygen therapeutics involve treatments that enhance oxygen delivery to tissues, addressing conditions like hypoxia and ischemia. Advances include the development of oxygen-carrying solutions and drugs that increase hemoglobin’s oxygen affinity or stimulate the production of erythropoietin, enhancing the body’s natural oxygen transportation capabilities. These innovations are paving the way for new treatments for diseases such as anemia, heart attack, and stroke, offering hope for improved outcomes and quality of life for patients.
Frequently Asked Questions
What is Hemoglobin?
Hemoglobin is a complex protein found in red blood cells, essential for transporting oxygen from the lungs to tissues throughout the body and returning carbon dioxide to the lungs for exhalation. It’s a key player in the respiratory process, ensuring that body cells receive the necessary oxygen to function properly and maintain vitality.
How Does Hemoglobin Carry Oxygen?
Hemoglobin carries oxygen through a process called oxygenation, where oxygen molecules bind to the iron atoms in hemoglobin’s structure. This binding transforms hemoglobin into its oxygenated form, enabling the transport of oxygen to body tissues. The oxygen is then released to the cells, supporting cellular metabolism and energy production.
Why is the Difference Between Oxygenated and Deoxygenated Hemoglobin Important?
The difference between oxygenated and deoxygenated hemoglobin is crucial for the efficient exchange of gases needed for respiration. Oxygenated hemoglobin delivers oxygen to tissues, while deoxygenated hemoglobin removes carbon dioxide, a byproduct of cellular metabolism. Understanding this difference is fundamental for diagnosing and treating conditions related to oxygen deficiency in the body, such as hypoxia.
How is Hemoglobin Oxygenation Measured?
Hemoglobin oxygenation is typically measured using pulse oximetry, a non-invasive method that estimates the percentage of oxygen-saturated hemoglobin in the blood. Blood gas analysis can also provide detailed information on hemoglobin oxygenation, including oxygen and carbon dioxide levels, which are critical for assessing a person’s respiratory and metabolic status.
Conclusion
The dynamic between oxygenated and deoxygenated hemoglobin is a testament to the complex and efficient mechanisms the body employs to sustain life. Through this intricate process, hemoglobin facilitates the essential exchange of oxygen and carbon dioxide, underpinning cellular respiration and energy production. This balance not only underscores the critical role of hemoglobin in our physiological processes but also highlights the importance of maintaining proper oxygen levels for overall health.
Understanding the nuances between oxygenated and deoxygenated hemoglobin provides valuable insights into the body’s respiratory efficiency and has significant implications for medical diagnostics and treatment. It emphasizes the necessity of maintaining optimal hemoglobin functionality to ensure the effective delivery of oxygen to tissues, a fundamental aspect of human health and well-being.