Osmium tetroxide and potassium permanganate stand as pillars in the realm of chemical reagents, each with unique properties that make them indispensable in both industrial applications and laboratory research. While they share common ground in their ability to act as oxidizing agents, the differences in their physical characteristics, chemical behavior, and applications set them apart. This distinction is crucial for chemists and engineers who rely on these compounds for precise and targeted reactions.
The core difference between osmium tetroxide and potassium permanganate lies in their chemical composition and the specific types of reactions they facilitate. Osmium tetroxide, with its heavy metal osmium center, is renowned for its role in organic synthesis, especially in the dihydroxylation of alkenes. On the other hand, potassium permanganate, a potent oxidizer, is widely used for its versatility in water treatment, disinfection, and as a reagent in analytical chemistry.
Both compounds exhibit distinct physical properties, such as appearance and solubility, and have unique safety protocols due to their high reactivity and potential health hazards. Understanding these differences is not only fundamental for safe and effective laboratory practices but also for optimizing industrial processes that depend on these chemicals’ specific properties.
Basic Properties
Osmium Tetroxide
Physical Characteristics
Osmium tetroxide (OsO4) is a compound with striking features. It appears as pale yellow crystals or a yellowish-brown powder at room temperature. Notable for its volatile nature, it readily sublimates, transitioning from a solid to a gas at temperatures above 40°C (104°F). This characteristic makes it somewhat unique among metallic compounds. Its density, being one of the highest among the compounds used in organic chemistry, sits at about 4.9 g/cm³. The compound is soluble in a variety of organic solvents, including acetone, alcohol, and chloroform, as well as being moderately soluble in water.
Chemical Behavior
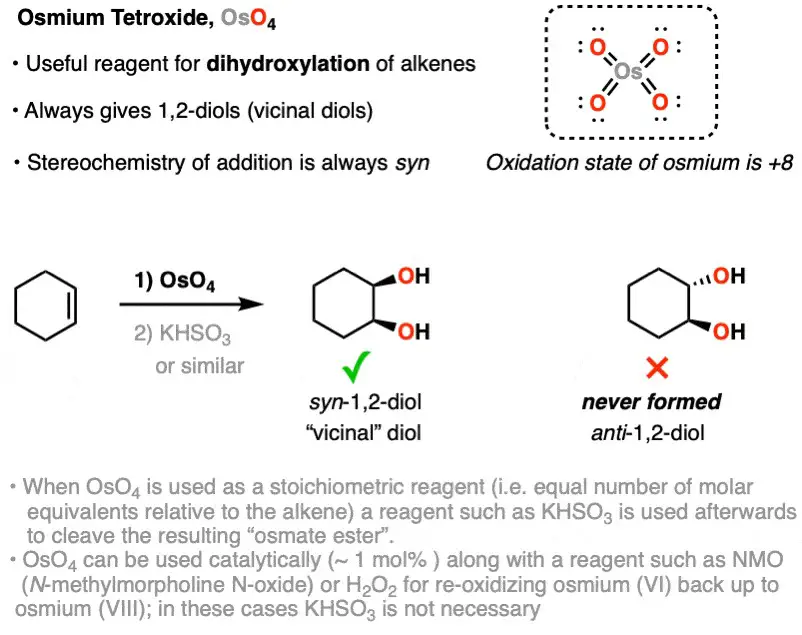
Osmium tetroxide is predominantly known for its role as a strong oxidizing agent. It has the unique ability to add two hydroxyl groups across the carbon-carbon double bond of alkenes, turning unsaturated compounds into glycols. This reaction is crucial in organic synthesis, providing a pathway to synthesize complex molecules. Unlike many oxidizing agents, OsO4 is selective and does not significantly over-oxidize, which makes it invaluable in delicate chemical manipulations.
Potassium Permanganate
Physical Characteristics
Potassium permanganate (KMnO4) presents as dark purple or blackish granular or needle-like crystals. Its bright purple solutions are a familiar sight in laboratories and various applications, indicating its strong oxidizing power. The compound is highly soluble in water, creating a purple solution that stains materials it comes into contact with. KMnO4’s solubility and the intense color of its solutions make it easily identifiable and widely used in chemical experiments and processes.
Chemical Behavior
KMnO4 is an aggressive oxidizing agent, capable of reacting with a wide range of organic and inorganic substances. In acidic, neutral, or alkaline media, it can undergo different chemical reactions, demonstrating its versatility. Among its many reactions, potassium permanganate can oxidize alcohols to ketones or carboxylic acids, sulfides to sulfates, and alkenes to diols, showcasing its broad utility in chemical synthesis and analytical chemistry. Its role as an indicator in titration reactions further emphasizes its importance in analytical applications.
Production Methods
Osmium Tetroxide
Extraction and Synthesis
The production of osmium tetroxide is not straightforward due to the rarity and high cost of osmium. It generally involves the oxidation of osmium metal with oxygen at elevated temperatures or through reaction with chlorine. The process is intricate and requires careful control of conditions to prevent loss of the volatile OsO4. Safety precautions are paramount due to the compound’s toxic nature.
Potassium Permanganate
Manufacturing Process
Potassium permanganate production typically follows a two-step process:
- Manganese dioxide is fused with potassium hydroxide and oxidized, usually with potassium nitrate or air, to form potassium manganate.
- The potassium manganate is then subjected to a controlled acidification process, which converts it to potassium permanganate. This step requires careful control to ensure the purity and yield of the final product.
The efficiency of the manufacturing process and the purity of the reactants are critical in determining the quality of the potassium permanganate produced.
Chemical Reactions
Osmium Tetroxide
Role in Organic Synthesis
Osmium tetroxide is renowned for its selective addition to alkenes, forming vicinal diols. This reaction, known as dihydroxylation, is vital for the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and polymers.
Specific Reaction Mechanisms
The mechanism involves the formation of a cyclic osmate ester intermediate, which is then hydrolyzed to yield the glycol. This process is highly selective and efficient, providing a crucial tool for chemists in the synthesis of complex molecules.
Potassium Permanganate
Oxidation Reactions
KMnO4 serves as a versatile oxidant in a plethora of reactions, capable of oxidizing alkenes to diols, alcohols to carbonyl compounds, and sulfides to sulfates. Its ability to act in different pH environments broadens its applicability across various chemical contexts.
Application in Analytical Chemistry
In addition to its use in synthesis, potassium permanganate is a staple in titration procedures to quantify the concentration of reducible substances. Its distinct color change upon reduction makes it an excellent indicator for the endpoint of titration reactions.
Safety and Handling
Osmium Tetroxide
Hazards and Precautions
Osmium tetroxide is highly toxic and volatile, posing significant inhalation risks. Handling requires:
- Adequate ventilation or use of a fume hood
- Protective clothing
- Tight-sealing containers for storage
Storage Guidelines
- Store in cool, dark places
- Ensure containers are well-sealed and labelled
Potassium Permanganate
Safety Concerns
KMnO4 is a strong oxidizer, which can cause fires if it comes into contact with flammable materials. It also poses risks of skin and eye burns upon contact.
Disposal Methods
- Neutralize with a reducing agent such as ascorbic acid before disposal
- Follow local regulations for disposal of hazardous materials

Industrial Applications
Osmium Tetroxide
Use in Microscopy
Osmium tetroxide plays a crucial role in electron microscopy as a stain. It binds to and stabilizes fatty acids and lipids, making them more visible under the microscope. This application is invaluable in cell biology and biochemistry, where understanding cell membrane structures and complex lipid distributions is essential.
- Staining Process: The compound reacts with unsaturated lipids, creating a contrast by turning them black. This enhances the visibility of biological samples.
- Advantages: Provides detailed images of cell structures, aiding in research and diagnostics.
Niche Applications
Beyond microscopy, osmium tetroxide has niche uses in synthetic organic chemistry and materials science.
- Synthetic Organic Chemistry: It is used for dihydroxylation reactions in the synthesis of complex organic molecules.
- Materials Science: Employed in the preparation of certain types of nanomaterials, due to its ability to oxidize and stabilize them.
Potassium Permanganate
Water Treatment
Potassium permanganate is a powerhouse in water treatment, used for its oxidizing properties to remove contaminants.
- Iron and Manganese Removal: Oxidizes dissolved iron and manganese for easier filtration.
- Disinfection: Kills bacteria and viruses, ensuring water is safe for consumption.
- Odor Control: Eliminates unpleasant odors by oxidizing organic compounds.
Medical Uses
In the medical field, KMnO4 finds applications as an antiseptic.
- Wound Cleaning: Diluted solutions are used to clean wounds and dermatitis.
- Fungal Infections: Treats certain fungal infections of the skin due to its fungicidal properties.
Environmental Impact
Osmium Tetroxide
Toxicity and Regulations
Osmium tetroxide’s high toxicity poses environmental and health risks.
- Environmental Hazards: Can cause damage to aquatic life if released into water bodies.
- Regulations: Strict handling and disposal regulations to minimize exposure and environmental impact.
Potassium Permanganate
Ecological Considerations
While KMnO4 is used in environmental applications, it must be managed carefully.
- Biodegradability: Not biodegradable, requiring careful disposal.
- Aquatic Toxicity: High concentrations can be toxic to aquatic organisms.
Cost and Availability
Osmium Tetroxide
Market Trends
The market for osmium tetroxide is niche, with its cost being relatively high due to its rarity and complex production process.
Factors Affecting Price
- Osmium Availability: Limited by osmium’s rarity and the demand in the market.
- Production Complexity: High costs associated with the extraction and synthesis of OsO4.
Potassium Permanganate
Global Production and Distribution
Potassium permanganate is widely produced and distributed, with its production being scaled to meet global demands.
- Manufacturing Hubs: Major producers include China, India, and Europe, contributing to its global availability.
- Distribution Channels: Widely available through chemical suppliers and online platforms.
Advantages and Limitations
Osmium Tetroxide
Unique Benefits
- Precision in Microscopy: Unmatched capability in enhancing cell structure visibility.
- Selective Oxidation: Ideal for sensitive synthetic chemistry applications.
Drawbacks in Use
- High Toxicity: Requires stringent safety measures.
- Cost: Prohibitively expensive for routine use in large-scale applications.
Potassium Permanganate
Versatility in Applications
- Broad-Spectrum Oxidant: Effective in a wide range of industrial, environmental, and medical applications.
- Indicator: Useful in analytical chemistry for titrations.
Challenges in Handling
- Strong Oxidizer: Risk of chemical burns and fires if not handled properly.
- Staining: Can leave purple stains on skin and materials, requiring careful handling.

FAQs
What is osmium tetroxide used for?
Osmium tetroxide is primarily used in organic chemistry for the dihydroxylation of alkenes, which adds two hydroxyl groups across the double bond, transforming it into a glycol. This reaction is crucial for synthesizing complex molecules in pharmaceuticals and organic compounds. Additionally, osmium tetroxide finds application in electron microscopy as a stain to enhance contrast by binding to specific cellular or material components.
How is potassium permanganate utilized in water treatment?
Potassium permanganate is a powerful oxidizing agent used in water treatment processes to remove iron and hydrogen sulfide from well water and waste water. It oxidizes dissolved iron and manganese, which then precipitate and can be filtered out. Potassium permanganate is also employed to control taste and odor in water and to disinfect water by killing bacteria and other pathogens.
Are osmium tetroxide and potassium permanganate dangerous?
Both osmium tetroxide and potassium permanganate are considered dangerous due to their high reactivity and potential health hazards. Osmium tetroxide is highly toxic, capable of causing severe eye, skin, and respiratory tract irritation, and long-term exposure can lead to lung damage. Potassium permanganate, while less toxic, can cause skin burns and respiratory issues if mishandled. Proper safety equipment and handling procedures must be strictly adhered to when working with these chemicals.
How do osmium tetroxide and potassium permanganate differ in chemical behavior?
Osmium tetroxide and potassium permanganate differ significantly in their chemical behavior. Osmium tetroxide acts specifically as an addition reagent in organic synthesis, particularly for the dihydroxylation of alkenes. Potassium permanganate, however, is a more general oxidizing agent that participates in a wide range of oxidation reactions, from organic synthesis to water treatment, making it versatile across various chemical processes.
Conclusion
In summary, osmium tetroxide and potassium permanganate serve as crucial chemical reagents in both laboratory research and industrial applications. Their unique properties and reactions highlight the importance of understanding their differences to harness their potential safely and effectively. Osmium tetroxide, with its specific application in organic synthesis, and potassium permanganate, with its broad utility in oxidation processes, exemplify the diversity and specificity of chemical reagents available to scientists and engineers.
The choice between osmium tetroxide and potassium permanganate depends on the specific requirements of the application at hand, underscoring the necessity for a thorough understanding of their properties, risks, and benefits. As we continue to explore and expand the boundaries of chemical science, these compounds will undoubtedly play pivotal roles in advancing our capabilities, from developing new pharmaceuticals to ensuring clean and safe water.

