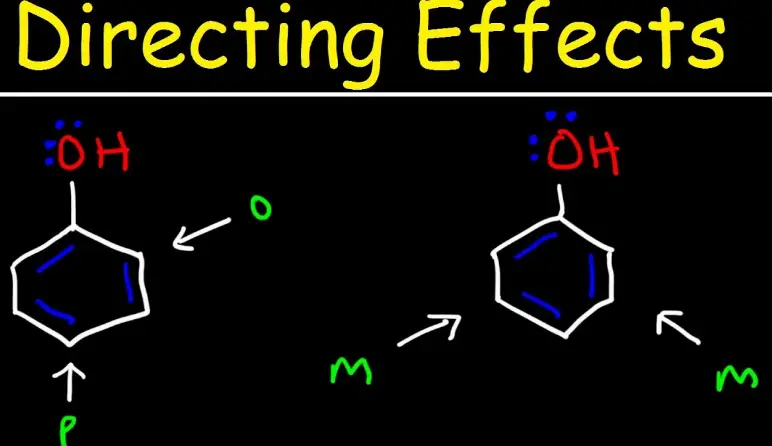
Electrophilic aromatic substitution is a cornerstone of organic chemistry, particularly in the synthesis involving benzene and its derivatives. This chemical process allows for the introduction of substituents into the aromatic ring, influencing the properties and reactivity of the resultant compounds. The positioning of these substituents is directed by existing groups on the benzene ring and is typically categorized into three types: ortho, para, and meta.
Ortho, para, and meta substitution refer to the positions where new substituents are added relative to an existing substituent on a benzene ring. Ortho and para substitutions occur adjacent to or directly opposite the existing group, respectively, enhancing the ring’s electron density and reactivity. In contrast, meta substitution happens at the position next to the adjacent one, usually resulting in decreased reactivity due to the electron-withdrawing effects of certain substituents.
These substitution patterns are not merely theoretical classifications; they are crucial for designing synthetic pathways in pharmaceuticals, dyes, and advanced materials. The specific position of a substituent can dramatically affect a molecule’s chemical behavior and, subsequently, its application potential. Recognizing the differences between these substitutions allows chemists to manipulate chemical structures purposefully to achieve desired properties and reactivity.
Aromatic Substitution Basics
Definition of Electrophilic Aromatic Substitution
Electrophilic Aromatic Substitution (EAS) is a chemical reaction where an electrophile replaces a hydrogen atom on an aromatic ring. This type of reaction is pivotal for modifying benzene rings, a fundamental component in many organic molecules used across various industries, from pharmaceuticals to plastics.
Role of Benzene Ring Stability
The stability of a benzene ring is central to its chemical behavior, especially during EAS. Benzene, characterized by a six-carbon ring with alternating double bonds, is exceptionally stable due to its aromaticity. This stability arises from the delocalized π-electrons across the ring, providing a robust platform for reactions that involve electrophiles without breaking the aromatic nature.
Types of Substituents
Activating vs. Deactivating
Substituents on a benzene ring can be classified based on their influence on reactivity and the type of substitution they favor:
- Activating substituents increase the reactivity of the benzene ring towards electrophilic attack. They are generally electron-donating groups such as -OH or -OCH₃, which push electron density into the ring through resonance and inductive effects.
- Deactivating substituents decrease the ring’s reactivity. These are typically electron-withdrawing groups like -NO₂ or -CF₃, pulling electron density away from the ring, making it less reactive towards electrophiles.
Electron-donating vs. Electron-withdrawing
The distinction between electron-donating and electron-withdrawing groups is crucial for predicting the outcome of EAS:
- Electron-donating groups (EDGs) donate electrons to the benzene ring, enhancing its electron density. Examples include amino (-NH₂) and methoxy (-OCH₃) groups.
- Electron-withdrawing groups (EWGs) remove electron density from the ring, which can inhibit reaction processes. Notable examples are nitro (-NO₂) and carbonyl (-COOH) groups.
Ortho Substitution
Definition and Characteristics
Ortho substitution occurs when a new substituent is introduced at a position adjacent to an existing substituent on the benzene ring. This type of substitution is favored by certain activating groups that enhance the electron density at the ortho positions through resonance stabilization.
Common Ortho-Directing Groups
Groups that direct electrophiles to the ortho positions typically include strong electron-donating groups such as:
- Methyl (-CH₃)
- Methoxy (-OCH₃)
- Amino (-NH₂)
These groups not only activate the ring but also influence the reaction pathway to favor substitution close to their location.
Examples in Synthesis
In practical synthesis, ortho substitution is used to produce a variety of chemical products:
- Anisole Production: Methylation of phenol with a methoxy group directs further methyl groups to the ortho position.
- Amino Acids Synthesis: Ortho-substituted derivatives are key intermediates in synthesizing certain amino acids.
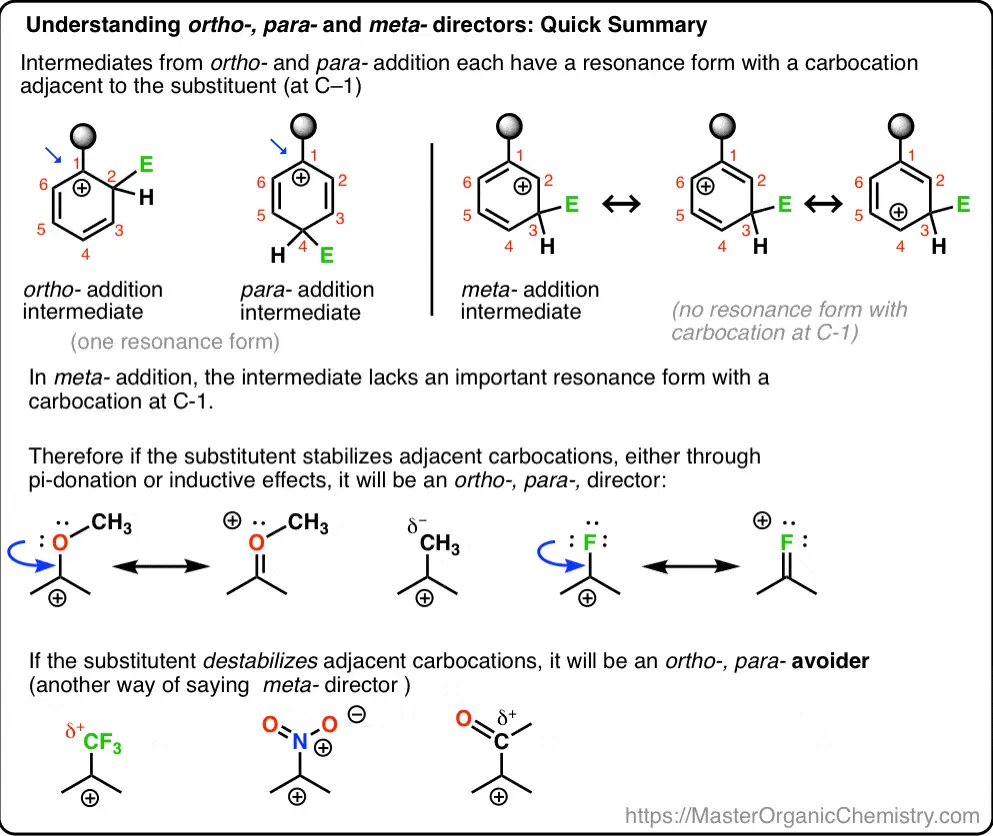
Para Substitution
Definition and Characteristics
Para substitution refers to the introduction of a substituent at the position directly opposite an existing group on the benzene ring. This pattern is often seen with substituents that stabilize the reactive intermediate by delocalizing charge across the aromatic system.
Common Para-Directing Groups
Para-directing groups are usually strong electron-donating groups that stabilize the transition state by resonance. These include:
- Hydroxyl (-OH)
- Amino (-NH₂)
Examples in Synthesis
Para-substituted compounds find extensive use in various applications:
- Paracetamol Production: The hydroxyl group in phenol directs the acetylation to the para position, forming paracetamol, a widely used pain reliever.
- Dye Manufacturing: Many dyes require para-substituted benzene derivatives for their vivid colors and stability.
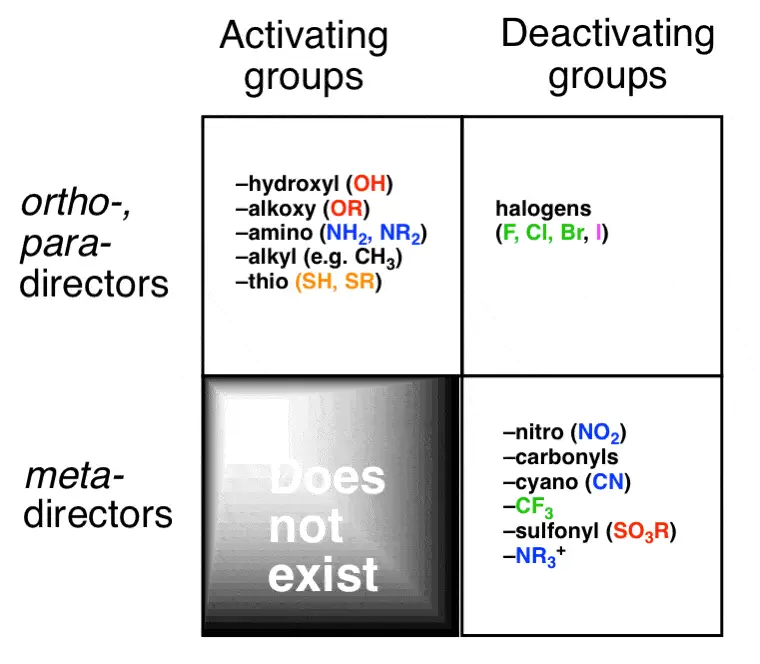
Meta Substitution
Definition and Characteristics
Meta substitution in aromatic chemistry occurs when an electrophile is introduced to the carbon atom located next to the adjacent carbon of an existing substituent. This position is less accessible compared to the ortho and para positions, which are more influenced by the presence of an electron-donating group. Meta positions are typically favored by substituents that are electron-withdrawing, making this type of substitution unique in its electronic requirements and outcomes.
Common Meta-Directing Groups
Electron-withdrawing groups (EWGs) such as nitro (-NO₂) and sulfonic acid (-SO₃H) are typical meta-directors. These groups stabilize the positive charge developed during the electrophilic attack far from themselves by withdrawing electron density through resonance and inductive effects.
Examples in Synthesis
Meta substitution is critical in the synthesis of many chemical compounds:
- Manufacturing of Dyes: Many synthetic dyes are structured around benzene derivatives that require precise meta substitution to achieve the desired hue and chemical properties.
- Pharmaceuticals: Certain drugs are synthesized using meta-substituted benzene rings to ensure proper spacing of functional groups, affecting the drug’s effectiveness and metabolic stability.
Comparison of Substitution Patterns
Factors Influencing Position
The position of substitution on the benzene ring is primarily influenced by:
- Nature of the substituent: Whether it is electron-donating or electron-withdrawing.
- Steric factors: The physical space around the substituent can hinder or facilitate incoming electrophiles.
- Resonance stability: Some positions offer more stable intermediates through resonance.
Chemical Reactions Comparisons
Understanding how ortho, para, and meta substitutions differ is crucial:
- Ortho and Para: These positions are often more reactive due to the stabilization from electron-donating groups.
- Meta: Typically less reactive unless influenced by strong electron-withdrawing groups.
Effects on Reactivity
Ortho and Para: Increased Reactivity
In reactions involving ortho and para substitutions, the presence of electron-donating groups significantly enhances the electron density of the aromatic ring, making it more reactive towards electrophiles. This increased reactivity is advantageous for synthesizing highly functionalized aromatic compounds efficiently.
Meta: Decreased Reactivity
Meta positions, conversely, often show decreased reactivity. This is because meta-directing groups are generally electron-withdrawing, which reduces the overall electron density of the aromatic ring, thus making it less appealing to electrophiles.
Practical Applications
Pharmaceuticals and Drug Synthesis
In the pharmaceutical industry, the precise control of substituent position on benzene rings can impact the pharmacological activity of a drug. Meta-substituted compounds often exhibit unique properties that can be critical for drug-receptor interactions.
Dyes and Pigments Manufacturing
The production of dyes and pigments often relies on specific substitution patterns to achieve desired colors and stability. Meta substitution is used to create compounds that offer specific light absorption properties essential for long-lasting dyes.
Challenges in Selective Substitution
Controlling Product Distribution
One of the primary challenges in aromatic substitution reactions is controlling the distribution of products:
- Selective Catalysts: Using catalysts that favor specific positions can help achieve higher selectivity.
- Temperature and Solvent Control: Adjustments in reaction conditions can sometimes influence the direction of substitution.
Advanced Techniques and Catalysts
To overcome these challenges, chemists employ advanced techniques and catalysts:
- Directed Metalation: Using metals like lithium or magnesium to direct the electrophile to the desired position.
- Computational Chemistry: Modeling and simulations help predict the outcomes of substitution reactions, guiding experimental approaches.
Frequently Asked Questions
What is Electrophilic Aromatic Substitution?
Electrophilic aromatic substitution is a reaction where an electrophile replaces a hydrogen atom on an aromatic ring. This fundamental mechanism is essential for adding various functional groups to benzene and other aromatic compounds, pivotal in synthesizing many commercial and pharmaceutical products.
How do Ortho, Para, and Meta Positions Affect Reactivity?
The ortho and para positions typically increase the reactivity of benzene rings due to their activating groups that enhance electron density. Conversely, meta positions often house deactivating groups that withdraw electron density, reducing the ring’s reactivity.
Why is Substituent Position Important in Synthesis?
The position of a substituent on a benzene ring critically influences the compound’s physical and chemical properties. Accurate positioning can dictate solubility, stability, and reactivity, crucial for effective drug design and material science applications.
Can Substituent Effects be Predicted?
Yes, substituent effects can often be predicted based on the type of group attached to the benzene ring. Activating groups generally direct new substituents to the ortho and para positions, while deactivating groups favor the meta position.
Conclusion
The distinctions between ortho, para, and meta substitutions form the basis for intricate chemical synthesis strategies across various industries. By understanding these differences, chemists can tailor organic molecules to exhibit specific properties, optimizing them for particular functions. This knowledge not only enhances the efficiency of synthetic routes but also expands the potential of aromatic compounds in developing novel materials and therapeutics.
The study of these substitution patterns also highlights the delicate interplay of electronic effects within aromatic rings, underscoring the profound impact of molecular positioning on chemical behavior. As research progresses, the precision in controlling these substitutions continues to grow, paving the way for more innovative and effective applications in science and industry.