Chromatography stands as a fundamental technique in scientific analysis, serving to separate mixtures into their individual components. This process is essential across various fields, from pharmaceuticals to environmental science, where precise component separation can be crucial for qualitative and quantitative analysis. Two predominant methods for achieving this are isocratic and gradient elution, each with unique benefits and challenges.
Isocratic elution maintains a constant solvent composition throughout the chromatographic process, making it straightforward and cost-effective for simpler mixtures. On the other hand, gradient elution varies the solvent strength gradually or in steps, allowing for more effective separation of complex mixtures over shorter times. The choice between these methods depends largely on the nature of the sample and the specific analytical requirements.
Both techniques are pivotal in modern chromatography, but their applications differ significantly based on the complexity of the samples and the precision required in the separation process. While isocratic elution offers simplicity and reproducibility, gradient elution provides flexibility and efficiency, making each method ideal under different circumstances.

Basics of Elution
Definition of Elution in Chromatography
Elution in chromatography refers to the process of passing a liquid or gas through a chromatography column to separate mixtures into their individual components. This phase is critical because it influences the effectiveness and efficiency of the separation process, directly affecting the analytical results. The solvent, known as the eluent, carries the mixture through the stationary phase, where different substances travel at different rates due to their varying chemical interactions with the stationary material.
Role in Separation Process
The role of elution in chromatography is pivotal as it determines how well components are separated from each other. The choice of eluent, its flow rate, and its composition all play essential roles in defining the separation quality. Effective elution leads to distinct peaks in the chromatogram, each representing a component from the original mixture, thereby allowing for precise identification and quantification.
What Is Isocratic Elution?
Core Concept
Isocratic elution is a technique in which the eluent’s composition remains constant throughout the chromatographic process. This method is particularly useful when the substances being separated have similar chemical properties and do not require a changing solvent strength to resolve their differences.
Typical Applications
Isocratic elution is commonly applied in routine testing where the compounds are well-known and do not require complex separation strategies. For instance, quality control in pharmaceutical manufacturing often relies on isocratic methods to ensure consistent product quality.
Benefits of Isocratic Elution
Simplicity and Stability
One of the main benefits of isocratic elution is its simplicity. With a fixed solvent composition, the system is easier to prepare and maintain, leading to more stable and reproducible results. This stability is crucial in high-throughput environments where time and accuracy are paramount.
Cost-effectiveness
Operating with a single solvent or a fixed solvent mixture reduces costs associated with solvent preparation and waste management. For laboratories mindful of budget constraints, isocratic elution offers an economical option without compromising analytical accuracy.
Suitability for Specific Analyses
Isocratic elution excels in scenarios where the analytes do not have a wide range of polarities. This makes it particularly suitable for substances that are similar in their chemical nature but still need to be quantified separately.
Challenges with Isocratic Elution
Limitations in Complex Mixtures
The major limitation of isocratic elution arises when analyzing complex mixtures. Since the solvent strength does not change, separating components with close retention times becomes challenging, often resulting in poor resolution.
Resolution Constraints
Isocratic elution may not adequately separate all components, especially in mixtures where the analytes have a broad range of chemical properties. This can lead to peak overlapping, making it difficult to distinguish between closely eluting substances.
What Is Gradient Elution?
Core Concept
Gradient elution contrasts with isocratic elution by varying the solvent composition during the chromatographic run. This approach adjusts the polarity of the solvent continuously or stepwise, enhancing the ability to separate analytes based on their interactions with the stationary phase.
How It Differs from Isocratic
Unlike isocratic elution, gradient elution can tackle complex samples with components that have significantly different chemical properties. By changing the solvent strength, it provides a more dynamic separation environment, which is crucial for resolving more complicated profiles.
Benefits of Gradient Elution
Enhanced Resolution
The ability to modify solvent strength in gradient elution means that each component in the mixture can be optimally separated. This dynamic adjustment leads to higher resolution and clearer separation of peaks in the chromatogram.
Flexibility in Applications
Gradient elution is versatile, accommodating a wide array of samples and complexities. It is particularly beneficial in research and development settings where unknown compounds may be present, or where the sample composition can vary significantly.
Time Efficiency
By optimizing the separation process through adjusted solvent strength, gradient elution often reduces the time required to achieve a complete separation. This efficiency is critical in high-throughput labs aiming to increase sample throughput without sacrificing quality.
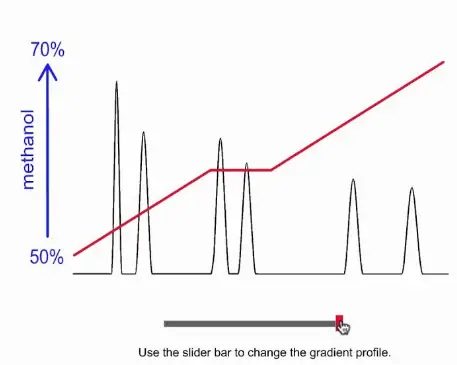
Challenges with Gradient Elution
Operational Complexity
Gradient elution is inherently more complex than isocratic elution due to its dynamic solvent changes. This complexity requires more sophisticated equipment and software to accurately control solvent gradients, increasing the skill level needed for operators. Technicians must understand how to adjust parameters like solvent ratios and gradient curves, which are crucial for achieving optimal separations.
Cost Implications
The use of multiple solvents and the need for advanced equipment make gradient elution a costlier option compared to isocratic elution. These costs are further amplified by higher solvent consumption and the need for regular maintenance of more complex systems. For laboratories working within tight budgets, these factors can be significant constraints.
Method Development
Developing methods for gradient elution can be time-consuming and requires extensive experimentation to optimize conditions for each analysis. Finding the right gradient profile involves balancing separation quality, analysis time, and resource usage, making method development a critical but resource-intensive phase.
Comparing Isocratic and Gradient
Direct Comparison of Methodologies
Isocratic elution offers simplicity and cost-effectiveness, making it ideal for routine analyses with known samples. In contrast, gradient elution provides superior resolution and adaptability, suited for complex or unknown samples. The choice between isocratic and gradient elution hinges on the specific requirements of the analysis, including the complexity of the sample and the precision needed in the results.
Decision Factors for Method Selection
When selecting an elution method, several factors should be considered:
- Sample complexity: More complex samples often require gradient elution for effective separation.
- Analysis time: Isocratic elution can be faster for simpler mixes, whereas gradient elution might be better for reducing overall run times with complex samples.
- Cost: Budget constraints may favor the less expensive isocratic approach.
- Equipment availability: The availability of sophisticated chromatography systems may influence the feasibility of implementing gradient elution.
Industries and Applications
Pharmaceuticals
In the pharmaceutical industry, both isocratic and gradient elution are employed for the analysis of raw materials, intermediates, and finished products. Gradient elution, in particular, is crucial for the detailed analysis required by regulatory standards, ensuring product safety and efficacy.
Environmental Testing
Environmental labs use chromatography to detect contaminants in water, soil, and air samples. Gradient elution is often necessary to resolve complex mixtures of environmental pollutants that require detailed characterization to meet regulatory guidelines.
Food and Beverage Analysis
Food safety testing relies on both isocratic and gradient elution to identify contaminants and ensure compliance with food safety standards. Gradient elution is particularly useful for analyzing complex ingredients or detecting trace levels of pesticides and additives.
Recent Advances
Technological Innovations
Recent advancements in chromatography equipment have enhanced both isocratic and gradient elution techniques. Innovations include more precise pumps and sophisticated software that allow for better control and reproducibility of gradient profiles, enhancing the overall quality of the separation process.
Improvements in Gradient Elution
Improvements in gradient elution technology have focused on increasing the speed and resolution of analyses. New column technologies and solvent management systems have been developed to handle faster solvent gradients, reducing analysis times while maintaining high resolution.
Case Studies
Practical Examples in Pharmaceuticals
A recent study in a pharmaceutical lab demonstrated the use of gradient elution to separate and quantify multiple active ingredients in a complex formulation, illustrating the technique’s ability to provide clear separation where isocratic methods were insufficient.
Environmental Analysis Showcase
In an environmental testing scenario, gradient elution was critical in resolving over 30 different pesticide residues in a single run from water samples. The use of a carefully optimized gradient ensured that all compounds were effectively separated and quantified within regulatory limits.
Frequently Asked Questions
What is isocratic elution?
Isocratic elution refers to a chromatographic technique where the solvent composition remains constant throughout the run. It’s particularly effective for analyzing compounds with similar characteristics, offering a simple, reproducible approach to separation.
What is gradient elution?
In gradient elution, the solvent composition changes during the chromatographic process. This method adjusts solvent strength gradually to more effectively separate complex mixtures, enhancing resolution and reducing run times.
When should I use isocratic elution?
Isocratic elution is best used when the mixture components have similar properties and do not require complex separation strategies. It is also preferred for routine analyses where cost and time efficiency are paramount.
When should I use gradient elution?
Gradient elution is ideal for complex mixtures with components that vary widely in their chemical properties. It is particularly useful in cases where high resolution and peak capacity are necessary for accurate analysis.
How do I choose between isocratic and gradient elution?
Choosing between isocratic and gradient elution depends on several factors including the complexity of the sample, the need for resolution, analysis time, and cost considerations. Understanding the specific requirements of your analytical procedure is key to selecting the appropriate elution method.
Conclusion
In conclusion, both isocratic and gradient elution play critical roles in the field of chromatography, tailored to meet different analytical needs. Isocratic elution, with its simplicity and cost-efficiency, suits straightforward, routine separations. In contrast, gradient elution, though more complex and potentially costly, offers enhanced capabilities for tackling challenging mixtures. The choice between these methods should be informed by the specific details of the sample and the analytical goals, ensuring optimal separation and accurate results.
By weighing the advantages and limitations of each technique, researchers and analysts can effectively decide the most appropriate approach, fostering advancements in scientific understanding and technological progress across various industries.

