Chromatography stands as a cornerstone technique in analytical chemistry, providing the means to separate complex mixtures into their individual components. This method leverages differences in the physical or chemical properties of compounds, facilitating their identification, quantification, and purification. Among the plethora of chromatographic techniques, ion pair and ion exchange chromatography are particularly noteworthy for their unique mechanisms and applications.
Ion pair chromatography involves the separation of molecules based on their interaction with a pair of ions added to the mobile phase, making it adept at analyzing neutral and ionic compounds alike. Ion exchange chromatography, on the other hand, separates ions based on their affinity to an oppositely charged stationary phase. This distinction is pivotal for scientists choosing a method for the separation of specific compounds, particularly in the fields of biochemistry and pharmaceuticals.
The intricacies of these methods lie in their operational parameters, such as mobile and stationary phases, and their application-specific advantages. Ion exchange chromatography excels in the purification of proteins and nucleic acids, thanks to its high selectivity and efficiency. Ion pair chromatography, with its versatile mobile phase modifications, shines in the realm of small molecules and complex biological samples analysis. Understanding these differences is crucial for effectively employing each technique to its fullest potential.
Chromatography Basics
Principles
Chromatography is a scientific technique used for the separation of a mixture into its individual components. This process leverages differences in the physical or chemical properties of the mixture’s constituents, allowing for their identification and analysis. At its core, chromatography involves passing a mixture dissolved in a mobile phase (usually a liquid or gas) through a stationary phase (a solid or a liquid supported on a solid). The components of the mixture interact differently with these phases, leading to their separation.
Types
Chromatography comes in various forms, each tailored to specific kinds of samples and analytical needs. Here’s an overview:
- Liquid Chromatography (LC): Separates mixtures with a liquid mobile phase.
- Gas Chromatography (GC): Utilizes a gas as the mobile phase.
- Thin Layer Chromatography (TLC): Involves a thin layer of stationary phase on a solid support.
- High-Performance Liquid Chromatography (HPLC): A more advanced form of liquid chromatography with higher resolution.
- Ion Exchange Chromatography (IEC) and Ion Pair Chromatography (IPC): Focus on the separation of molecules based on ionic interactions.
Each type serves distinct purposes, from simple qualitative analysis in TLC to complex quantitative analysis in HPLC.
Ion Exchange Chromatography
Core Concept
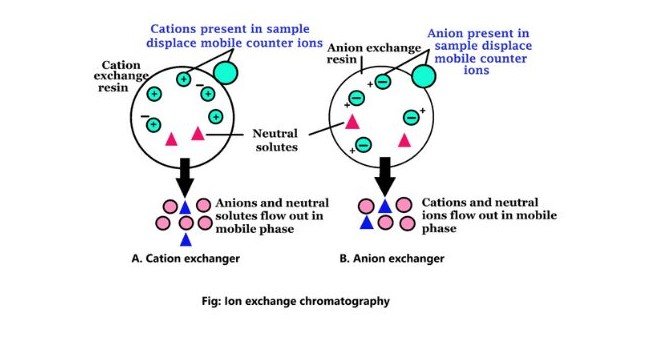
Ion exchange chromatography is a powerful method used to separate ions and polar molecules. It exploits the electrostatic forces between the ions in the sample and the ions attached to the stationary phase. This technique is widely used for its specificity, efficiency, and ability to purify compounds based on their charge.
Mechanism
The mechanism of ion exchange chromatography is straightforward yet elegant:
- The sample is introduced into the column containing a stationary phase with charged groups.
- Ions in the sample interact with these charged groups, replacing ions already bound to the stationary phase in a process known as ion exchange.
- Components are eluted (washed out) using a buffer. The strength of the buffer or its ionic concentration can be adjusted to control the elution order of the ions.
This method’s efficiency lies in its ability to selectively separate ions based on their charge and size.
Applications
Ion exchange chromatography is indispensable in both research and industry. Its applications include:
- Purification of proteins, including enzymes and antibodies.
- Separation of nucleic acids (DNA and RNA).
- Water purification and softening processes.
- Drug development and quality control in the pharmaceutical industry.
Ion Pair Chromatography
Core Concept
Ion pair chromatography stands out for its versatility in analyzing both neutral and ionic compounds. It adds a unique component to the mobile phase, known as an ion pairing reagent, which pairs with the ions in the sample. This pairing alters the elution characteristics of the ions, facilitating their separation based on hydrophobic interactions and molecular size.
Mechanism
The underlying mechanism of ion pair chromatography involves several key steps:
- Addition of an ion pairing reagent to the mobile phase.
- Formation of ion pairs between the reagent and the components of the sample.
- Separation of these ion pairs on a chromatographic column, typically one designed for reverse-phase chromatography.
The choice of ion pairing reagent is crucial, as it determines the interaction strength and thus the separation efficiency.
Applications
Ion pair chromatography is particularly useful for the separation of compounds that are difficult to distinguish by other methods. Its applications span:
- Analysis of pharmaceuticals, including detection of drug metabolites.
- Separation of biological molecules like peptides, nucleotides, and proteins.
- Environmental analysis, such as the detection of pollutants in water.
Comparing Techniques
Mobile Phase
The mobile phase in chromatography plays a pivotal role in the separation process, acting as the carrier of the sample through the stationary phase. The selection of the mobile phase is crucial, as it directly affects the efficiency and effectiveness of the separation.
Ion Exchange Chromatography typically utilizes water or aqueous buffers as the mobile phase. The choice of buffer, its pH, and ionic strength are critical factors that influence the separation process. These parameters must be carefully optimized to achieve the desired interaction between the analytes and the stationary phase.
Ion Pair Chromatography, on the other hand, requires the addition of an ion pairing agent to the mobile phase. This agent, often a surfactant, interacts with the analytes to form ion pairs. The composition of the mobile phase, including the type and concentration of the ion pairing agent, is essential for modulating the retention time of the analytes on the column.
Stationary Phase
The stationary phase is the heart of the chromatographic separation, providing the surface or medium upon which the separation of compounds is based.
In Ion Exchange Chromatography, the stationary phase is composed of a resin or polymer that carries charged functional groups. These charged groups interact with oppositely charged ions in the sample. The nature of these interactions can be cationic or anionic, depending on the type of ion exchange chromatography being employed.
Ion Pair Chromatography uses a stationary phase that is typically non-polar or moderately polar. The ion pairing agents added to the mobile phase interact with the analytes, altering their effective polarity or charge. This modified interaction between the analytes and the stationary phase facilitates the separation process.
Sample Preparation
Sample preparation is a critical step in achieving successful chromatographic separation, with each technique requiring specific considerations.
For Ion Exchange Chromatography, the sample must be in a suitable buffer that matches the pH and ionic strength requirements of the column. Samples may also need to be desalted or diluted to fit the optimal conditions for separation.
In Ion Pair Chromatography, the sample preparation might involve adjusting the concentration of the ion pairing agent in the sample solution to ensure consistent interaction with the analytes. This preparation step is crucial for maintaining the stability of the ion pairs during the separation process.
Sensitivity and Selectivity
Sensitivity and selectivity are key performance indicators in chromatography, determining the method’s ability to detect and separate compounds within a mixture.
Ion Exchange Chromatography is highly selective for charged molecules, with sensitivity that can be adjusted through the manipulation of the mobile phase’s ionic strength and pH. This technique is particularly effective for separating compounds with slight differences in charge.
Ion Pair Chromatography offers enhanced sensitivity and selectivity for neutral and ionic compounds by controlling the concentration and type of ion pairing agent. This method is capable of separating compounds with similar structures or properties that would otherwise co-elute.
Advantages and Limitations
Ion Exchange Chromatography
Advantages:
- High selectivity for charged molecules
- Capable of purifying large biomolecules such as proteins
- Can be easily scaled up for industrial applications
Limitations:
- Less effective for neutral molecules
- Requires careful control of mobile phase conditions
- Potential for sample loss or denaturation during the separation
Ion Pair Chromatography
Advantages:
- Versatile, capable of separating a wide range of compounds
- Effective for compounds that are difficult to separate by charge alone
- Allows for the modulation of retention times through the mobile phase composition
Limitations:
- Requires the use of ion pairing agents, which can complicate the mobile phase preparation
- Potential interference from the ion pairing agent in the detection of analytes
- May require additional steps to remove the ion pairing agent from the sample post-separation
Selecting the Right Method
Choosing between Ion Exchange and Ion Pair Chromatography depends on several factors:
- Nature of the Sample: Charged biomolecules might favor ion exchange, while neutral or similarly charged compounds might be better suited for ion pair chromatography.
- Resolution Requirements: The specificity of separation needed can influence the choice.
- Scale of Operation: Considerations for scale-up can affect the decision, especially in industrial applications.
Recent Advances
Recent advancements in chromatography have focused on improving efficiency, resolution, and the ability to separate complex mixtures.
In Ion Exchange Chromatography, innovations include the development of new resin materials with higher capacities and selectivities. These advancements allow for the more efficient separation of proteins and other biomolecules.
Ion Pair Chromatography has seen the introduction of new ion pairing agents and improved stationary phases designed to enhance separation capabilities and reduce analysis times.
Frequently Asked Questions
What is ion exchange chromatography?
Ion exchange chromatography is a process that allows the separation of ions and polar molecules based on their affinity to ion exchangers. It relies on the reversible interactions between charged sample ions and oppositely charged ions fixed on a stationary phase. This method is particularly effective for purifying proteins, peptides, and nucleic acids, offering high resolution and selectivity.
How does ion pair chromatography work?
Ion pair chromatography operates by adding ion pairing agents to the mobile phase, which bind to the analyte of interest. This binding changes the analyte’s apparent polarity or charge, enabling its separation on a chromatographic column. The technique is versatile, suitable for separating a wide range of compounds, from small organic molecules to large biomolecules.
When should I choose ion exchange over ion pair chromatography?
The choice between ion exchange and ion pair chromatography depends on the nature of the sample and the separation goals. Ion exchange chromatography is ideal for separating charged biological molecules, such as proteins and nucleic acids, due to its high specificity and capacity for purification. Ion pair chromatography is preferred for compounds that are difficult to separate by charge alone, including neutral molecules and those with similar charge properties.
Conclusion
Deciphering the nuances between ion pair and ion exchange chromatography is not just academic; it’s a critical decision point in the workflow of countless scientific endeavors. Each method offers distinct advantages and limitations, tailored to specific types of analytes and separation challenges. The choice hinges on the chemical nature of the compounds under investigation and the desired purity and resolution.
This exploration into the realms of ion pair and ion exchange chromatography underscores the importance of analytical techniques in advancing scientific research and industrial applications. By selecting the appropriate chromatographic method, researchers can unlock the full potential of their analyses, paving the way for new discoveries and innovations. In the end, the mastery of these techniques enriches our understanding of the molecular world, one separation at a time.

