Complexes in chemistry are fascinating entities composed of molecules formed by a central metal atom bound to surrounding groups or molecules known as ligands. This binding can result in a diverse array of properties, which are pivotal in numerous applications, from industrial catalysis to medicinal treatments. The behavior and characteristics of these complexes vary significantly depending on their stability and reactivity.
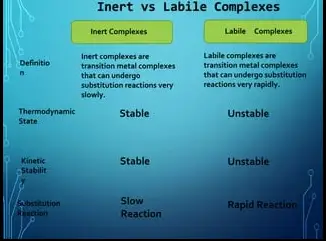
The difference between inert and labile complexes lies primarily in their reaction kinetics. Inert complexes are stable and undergo reactions at a slower rate, making them less reactive under normal conditions. On the other hand, labile complexes react quickly and are generally less stable. This distinction plays a crucial role in their practical applications and influences how they are utilized in various chemical processes.
Understanding these differences is not only essential for chemists but also for industries that rely on these complexes for precise reactions and outcomes. The balance of stability and reactivity in these complexes can affect everything from the speed of a reaction to the longevity and effectiveness of a catalyst in industrial processes.
Chemical Composition
Elements of a Complex
Complexes, fundamental entities in coordination chemistry, are comprised of a central metal atom or ion and surrounding ligands. These ligands are molecules or ions that donate a pair of electrons to the metal, forming a coordinate covalent bond. This interaction is crucial as it defines the structure and properties of the complex.
Bonding Characteristics
The bonding in complexes is characterized by the dative covalent bonds between the metal and the ligands. The nature of these bonds significantly influences the overall stability and reactivity of the complex. Important factors include the electron configuration of the metal, the type of ligands, and the geometry of the complex which can be tetrahedral, square planar, octahedral, and others.
Inert Complexes
Characteristics
Inert complexes are noted for their stability and low reactivity. These complexes do not readily undergo ligand substitution reactions, making them stable over time under various conditions. This stability is attributed to strong metal-ligand bonds and is often desired in applications where prolonged activity without degradation is crucial.
Stability Factors
Several factors contribute to the stability of inert complexes:
- High bond dissociation energy making the breaking of metal-ligand bonds difficult.
- Low reorganization energy, where the complex does not require significant energy to reorganize its structure for a reaction.
- Denticity of the ligands, where multidentate ligands (those that can form multiple bonds) create more stable complexes through the chelate effect.
Common Examples
Examples of inert complexes include:
- [Co(NH₃)₆]³⁺, a cobalt complex with ammonia which is used in biochemical separations.
- [Fe(CN)₆]³⁻, used in electroplating and as a redox agent.
Formation
Kinetics of Formation
The formation of inert complexes often involves slow reaction kinetics:
- Stepwise addition of ligands, where each step can be slow and controlled.
- High activation energy for the formation, which prevents rapid formation and degradation.
Influencing Factors
Factors influencing the formation include:
- Temperature, where lower temperatures generally favor the formation of more inert complexes.
- Concentration of ligands, which can affect how quickly the complex forms.
Labile Complexes
Characteristics
Labile complexes are characterized by their high reactivity and instability. These complexes can rapidly exchange their ligands even in mild conditions, which makes them suitable for reactions requiring fast kinetics.
Instability and Reactivity
The instability is often a result of:
- Weak metal-ligand bonds, which easily break and reform.
- High reorganization energy, where the complex readily changes its structure to facilitate reactions.
Common Examples
Common examples of labile complexes include:
- [Cu(H₂O)₆]²⁺, a copper complex used in catalysis.
- [Ni(H₂O)₆]²⁺, used in hydration reactions.
Formation
Formation Speed
Labile complexes form quickly, often in:
- Rapid ligand exchange processes, where ligands can be replaced in a matter of seconds or minutes.
- Low activation energy pathways, facilitating faster reactions.
Influencing Factors
Factors influencing the speed of formation include:
- Solvent type, which can increase or decrease the rate of ligand exchange.
- pH of the solution, affecting the stability and reactivity of the metal ion.
Comparative Analysis
Stability Comparison
In comparing inert and labile complexes, stability is primarily determined by:
- Ligand strength, with stronger field ligands typically leading to more stable, inert complexes.
- Metal ion characteristics, where transition metals with a high oxidation state usually form more stable complexes.
Role of Ligands
Ligands are crucial in determining the stability and reactivity of complexes. Strong field ligands, like CN⁻ and CO, generally form more stable complexes due to their ability to donate electrons more effectively to the metal.
Electronic Configurations
The electronic configuration of the metal ion plays a significant role. Metals with d⁰ or d¹⁰ configurations tend to form more labile complexes due to their lack of available d-orbitals for bonding, leading to weaker interactions with ligands.
Reaction Pathways
Mechanisms of Change
The mechanisms through which complexes undergo changes include:
- Ligand substitution, where one ligand is replaced by another.
- Redox reactions, involving changes in the oxidation state of the metal.
Environmental Impact
The use of metal complexes can have significant environmental impacts. Inert complexes, due to their stability, are less likely to release metals into the environment, while labile complexes, due to their reactivity, might contribute to metal mobility and potential toxicity.
Applications and Implications
Industrial Applications
Complexes, particularly inert and labile ones, play pivotal roles in various industries. Their ability to catalyze reactions, bind with other molecules, and facilitate or inhibit processes makes them invaluable across multiple sectors.
Usage in Catalysis
In the field of catalysis, complexes are indispensable. For instance, inert complexes are used in hydrogenation reactions where their stability under high-pressure and temperature conditions is crucial. These complexes facilitate the addition of hydrogen to other molecules, often in the creation of fuels or the reduction of carbon double bonds in organic compounds. Labile complexes, on the other hand, are favored in environments where fast reaction times are essential. They are commonly used in bulk chemical production where rapid catalyst regeneration is necessary to maintain efficiency.
Medicinal Relevance
In medicine, metal complexes serve as agents in diagnostic imaging and drug delivery systems. For example, Gd(III) complexes are used in MRI imaging to enhance the contrast, making it easier to view soft tissues. The rapid response of labile complexes to changing conditions in the body makes them excellent candidates for delivering drugs to specific sites within the organism.
Environmental Impact
The use of metal complexes also extends to environmental applications, where their reactivity and stability can significantly influence ecological outcomes.
Ecosystem Interactions
Metal complexes can interact with ecosystems in ways that both benefit and harm environmental stability. For example, copper complexes used in pesticides help control harmful pests but can also lead to copper accumulation in the soil, which might affect microbial communities and plant health.
Pollution Considerations
The stability of inert complexes can be a double-edged sword. While they are less likely to break down and release metals into the environment, if they do accumulate, they can become long-lasting pollutants. On the flip side, labile complexes, due to their reactivity, might break down more quickly but can release metals in more bioavailable forms, which might be more toxic to wildlife.
Future Research Directions
As we look to the future, the study and application of inert and labile complexes are bound to intersect with emerging technologies and lead to new discoveries.
Emerging Technologies
One of the most promising areas of research involves the integration of metal complexes with nanotechnology. For example, using inert metal complexes to stabilize nanoparticles can lead to innovations in the delivery of pharmaceuticals and even in the design of new types of solar cells where these complexes help in the efficient conversion of solar energy.
Potential Discoveries
The frontier of metal complex research also extends into the realm of quantum computing and energy storage. Metal complexes that can exhibit quantum properties at higher temperatures could revolutionize the field of quantum computing. Moreover, labile complexes are being studied for their potential in high-density energy storage systems, where their ability to rapidly change states can be harnessed to improve battery efficiency and capacity.
Frequently Asked Questions
What Are Inert Complexes?
Inert complexes are those that exhibit low reactivity due to strong and stable bonds between the central metal and its ligands. These complexes typically have slow exchange rates for ligands and are often used in conditions where consistent performance over time is necessary.
What Are Labile Complexes?
Labile complexes are characterized by their high reactivity, which is attributed to weaker bonds between the central metal and its ligands. These complexes can rapidly change their structure and ligands, making them useful in processes that require quick reactions.
Why Is Stability Important in Complexes?
Stability in complexes determines their reactivity and durability under various chemical conditions. Stable, inert complexes are crucial in applications requiring long-term consistency, whereas labile complexes are preferred in fast-paced reactions where quick transformation is beneficial.
How Do Ligands Affect Complex Stability?
Ligands play a pivotal role in defining the stability of complexes. Strong field ligands can enhance stability by providing stronger bonding interactions, leading to more inert characteristics. Conversely, weak field ligands often result in more labile complexes due to their less robust bonding nature.
Conclusion
Complexes play an integral role in the field of chemistry, influencing various industrial and medicinal applications through their unique properties of stability and reactivity. The distinction between inert and labile complexes, dictated by their kinetic behaviors, informs their suitability for specific applications, making an understanding of their differences fundamental for chemists and industry professionals alike.
In sum, the study of inert versus labile complexes is more than an academic endeavor—it’s a crucial component of advancing practical chemical applications. By leveraging the stability of inert complexes and the reactivity of labile ones, scientists and engineers can optimize processes to achieve desired outcomes more efficiently and effectively.