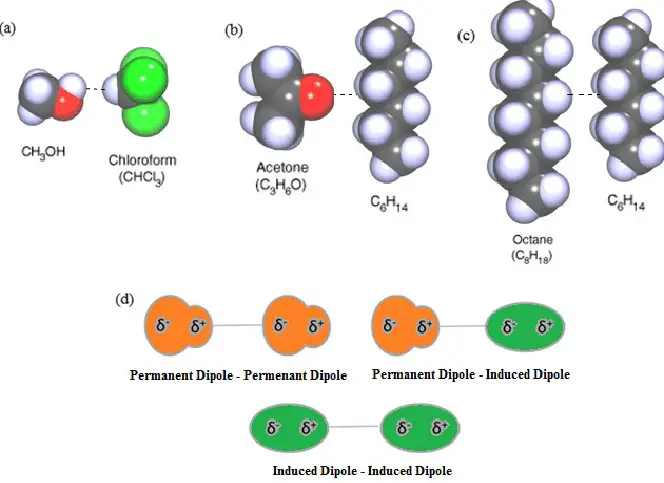
Molecular dipoles are a fundamental aspect of chemistry and physics, underlying many of the properties and behaviors of materials we encounter daily. At the heart of molecular interactions, the concept of dipoles explains everything from the water’s ability to dissolve common salts to the intricacies of biological molecules shaping life itself. Dipoles are not just abstract concepts but are observable phenomena with profound implications on the molecular scale.
The difference between induced dipole and permanent dipole lies primarily in their formation and stability. A permanent dipole results from the inherent molecular structure, where there is an uneven distribution of electron density across the molecule. On the other hand, an induced dipole occurs when the electron distribution of a molecule is temporarily distorted by the electrical influence of a neighboring molecule or an external electric field, leading to a momentary dipole.
Understanding these dipoles provides insight into the molecular world’s complexity, where forces invisible to the naked eye dictate the behavior of substances. From how materials interact to the design of new drugs, the distinction between induced and permanent dipoles is a key piece of the puzzle in unraveling the mysteries of chemistry and physics. It’s a fascinating realm where microscopic interactions have macroscopic consequences, influencing everything from material science to our understanding of the natural world.
Dipole Basics
What is a Dipole?
A dipole is a concept in physics and chemistry that describes the separation of charges within a molecule. Imagine a magnet with a north and south pole; similarly, a dipole has a positive charge on one end and a negative charge on the other. This separation of charge leads to a molecular polarity, a key factor that influences how molecules interact with each other and their surroundings.
Types of Dipoles
Dipoles are divided into two main categories: permanent dipoles and induced dipoles.
- Permanent dipoles are the result of the molecular structure where atoms share electrons unevenly.
- Induced dipoles occur when an external force, such as an electric field, temporarily distorts the electron cloud of a molecule.
Permanent Dipole
Definition and Formation
A permanent dipole forms in a molecule due to the unequal sharing of electrons between atoms. This inequality is because atoms have different electronegativities, a measure of how strongly they attract electrons. When two atoms with different electronegativities form a bond, the electrons tend to be more attracted to one atom, leaving it partially negative and the other atom partially positive.
Molecular Structure Influence
The molecular structure plays a crucial role in the formation of a permanent dipole. The shape of the molecule and the orientation of its bonds determine whether the individual bond dipoles cancel out or add up to create a net dipole moment.
Characteristics
Polarity
The polarity of a molecule is a direct result of its permanent dipole. Polar molecules have a significant difference in electronegativity between their atoms, leading to a strong dipole moment.
Examples in Nature and Industry
- Water (H2O): Perhaps the most famous example, water’s unique properties, such as its solvent capabilities, are due to its permanent dipole.
- Hydrogen Chloride (HCl): Used in industrial processes, its dipole nature makes it an excellent acid.
Induced Dipole
Definition and Formation
An induced dipole occurs when the electron distribution of a non-polar molecule is distorted by the electric field of another molecule or an external source. This distortion creates a temporary dipole within the molecule, affecting its interactions without altering its permanent structure.
Influence of External Electric Fields
The presence of an electric field, whether from a nearby polar molecule or an artificial source, can shift the electron cloud in a non-polar molecule, inducing a dipole. The strength of the induced dipole is proportional to the strength of the electric field.
Characteristics
Temporary Nature
Induced dipoles are temporary; they exist only as long as the external electric field is present. Once the field is removed, the electron cloud returns to its original, symmetrical distribution.
Examples in Everyday Phenomena
- Van der Waals Forces: Induced dipole-induced dipole interactions contribute to the forces that allow geckos to adhere to surfaces.
- Non-polar Solvents: These can dissolve non-polar substances thanks to the temporary dipoles induced by molecular interactions.
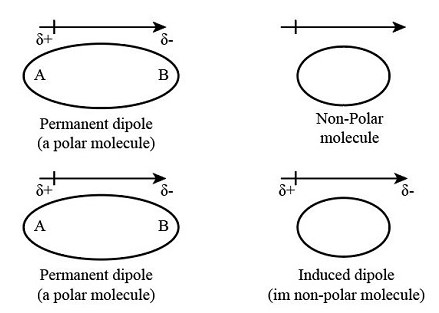
Key Differences
Structural Origins
Molecular Geometry vs. External Influence
The core difference between permanent and induced dipoles lies in their origin. Permanent dipoles arise from the molecular geometry itself. This geometry leads to an uneven distribution of electrons, making one part of the molecule more negative and another more positive. In contrast, induced dipoles result from an external influence, such as the presence of a polar molecule or an electric field that temporarily shifts the electron cloud within a molecule.
Duration and Stability
Temporary vs. Stable
Permanent dipoles are stable; they exist as long as the molecule exists. Their stability is a result of the molecular structure and does not change unless the molecule undergoes a chemical reaction. On the other hand, induced dipoles are temporary. They last only as long as the external influence persists. Remove the influence, and the molecule reverts to its original, non-polar state.
Impact on Properties
Boiling Points, Solubility, and Reactivity
Dipoles significantly affect a molecule’s boiling point, solubility, and reactivity.
- Boiling Points: Molecules with permanent dipoles generally have higher boiling points due to the stronger intermolecular forces.
- Solubility: Solubility is influenced by the “like dissolves like” principle, where polar substances dissolve better in polar solvents due to dipole-dipole interactions, and non-polar substances dissolve in non-polar solvents due to induced dipole-induced dipole interactions.
- Reactivity: The presence of a dipole can enhance a molecule’s reactivity, especially in polar solvents, as the polar environment can stabilize transition states and intermediates.
Role in Chemical Reactions
Solvation Process
Interaction with Solvents
The solvation process is a key area where dipoles play a crucial role. When a solute dissolves in a solvent, the dipoles of the solvent molecules interact with the solute particles. This interaction, whether through dipole-dipole forces or induced dipole forces, is fundamental to the dissolution process, affecting the solubility of compounds.
Intermolecular Forces
London Dispersion Forces vs. Dipole-Dipole Interactions
Two main types of intermolecular forces involve dipoles: London dispersion forces and dipole-dipole interactions. London dispersion forces are a type of induced dipole force that occurs even between non-polar molecules, while dipole-dipole interactions are the result of the electrostatic attraction between the positive end of one polar molecule and the negative end of another. Dipole-dipole interactions are generally stronger and play a significant role in determining the physical properties of substances.
Applications and Implications
In Pharmaceuticals
Drug Design and Solubility
In the pharmaceutical industry, understanding dipoles is critical for drug design and solubility. The polarity of a molecule can influence its ability to interact with biological targets, its solubility in bodily fluids, and its overall efficacy as a drug. Designing molecules with the right balance of polar and non-polar characteristics can enhance drug delivery and effectiveness.
In Material Science
Polymer Behavior and Electronics
The study of dipoles has significant implications in material science, particularly in the behavior of polymers and the development of electronic materials. Polymers with polar groups can exhibit different physical properties, such as increased toughness or improved electrical conductivity. Similarly, in electronics, the alignment of dipoles in materials can affect their dielectric properties, crucial for capacitors and other components.
Environmental Impact
Atmospheric Particles and Pollutants
Dipoles also have an environmental impact, particularly in the behavior of atmospheric particles and pollutants. The interaction of polar molecules with water vapor can influence cloud formation and weather patterns. Furthermore, the solubility of pollutants in water bodies is affected by their polarity, which can determine their distribution, persistence, and toxicity in the environment.
Frequently Asked Questions
What is a Dipole Moment?
A dipole moment is a measure of the separation of positive and negative charges in a system, indicating the polarity of a molecule. It’s a vector quantity with both magnitude and direction, reflecting how strongly polar a bond is. In permanent dipoles, this moment is constant, determined by the molecular structure. In induced dipoles, the moment is temporary, arising under the influence of external electric fields or neighboring molecules.
How Do Dipoles Affect Boiling Points?
Dipoles significantly influence a substance’s boiling point. Molecules with permanent dipoles tend to have higher boiling points due to the stronger dipole-dipole interactions that require more energy to overcome. Induced dipoles contribute to London dispersion forces, which are weaker than dipole-dipole interactions but become significant in larger, more polarizable molecules, subtly increasing boiling points.
Can Induced Dipoles Become Permanent?
Induced dipoles cannot become permanent as they are a result of temporary distortions in the electron cloud of molecules due to an external force or the presence of another molecule. Once the external influence is removed, the molecule will revert to its original state. The permanency of a dipole is intrinsic to the molecule’s structure and cannot be altered by temporary external factors.
Why Are Dipoles Important in Solvents?
Dipoles play a crucial role in the effectiveness of solvents. Solvents with a permanent dipole can dissolve polar substances effectively through dipole-dipole interactions. Similarly, the ability of non-polar solvents to induce dipoles allows them to dissolve non-polar substances through induced dipole-induced dipole interactions. This principle is crucial in designing solvents for specific applications, from pharmaceuticals to industrial processes.
Conclusion
The interplay between induced dipoles and permanent dipoles is a testament to the nuanced and intricate nature of molecular interactions. These interactions are not merely academic concepts but are the linchpins in the vast machinery of the physical world, dictating the properties and behaviors of substances. Understanding the distinction between these two types of dipoles unlocks a deeper appreciation for the complexity of the molecular world and its profound impact on the macroscopic level.
Recognizing the fundamental differences between induced and permanent dipoles equips us with the knowledge to predict and manipulate the physical and chemical properties of substances. This understanding is invaluable across a spectrum of fields, from pharmaceuticals to material science, showcasing the unity of knowledge across disciplines. As we continue to explore and harness the intricacies of dipoles, we pave the way for innovative solutions to some of the world’s most pressing challenges, underlining the enduring relevance of this fundamental scientific principle.