Metallurgical processes form the backbone of the materials science industry, enabling the extraction and refinement of metals from their ores. These methods, essential for manufacturing and technology development, fall into two primary categories: hydrometallurgy and pyrometallurgy. Each process employs distinct principles and methodologies to recover valuable metals, playing a pivotal role in the global supply chain of raw materials.
Hydrometallurgy involves extracting metals from their ores through aqueous chemistry. This method uses solvents to dissolve metal compounds, facilitating their separation and recovery at ambient or slightly elevated temperatures. Conversely, pyrometallurgy relies on high-temperature treatments such as roasting, smelting, and refining to extract and purify metals. These thermal processes transform ores into molten metals, allowing for the separation of valuable elements from impurities.
The choice between hydrometallurgy and pyrometallurgy hinges on various factors, including the nature of the ore, environmental considerations, energy consumption, and cost. Hydrometallurgy is often preferred for its lower environmental impact and ability to process low-grade ores efficiently. Meanwhile, pyrometallurgy is favored for its high throughput and efficiency in processing ores with higher metal content. The strategic selection of either method significantly influences the sustainability and profitability of metal extraction and refining operations.

Basic Principles
Hydrometallurgy Overview
Definition and Process
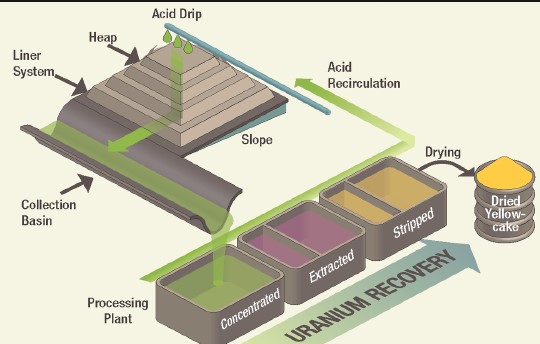
Hydrometallurgy is a branch of metallurgy that involves extracting metals from their ores using aqueous solutions. This method is particularly suitable for ores that are not amenable to direct extraction by pyrometallurgical processes. The core advantage of hydrometallurgy lies in its lower environmental impact and its ability to efficiently process low-grade ores.
- Leaching: The process begins with leaching, where an acidic or basic solution is used to dissolve the metal ions from the ore.
- Separation and concentration: The leachate containing the metal ions is then separated from the solid waste, known as tailings, and concentrated.
- Metal recovery: Finally, the metal is recovered from the solution through various methods, such as precipitation, adsorption, or electrolytic refining.
Key Components and Chemicals Used
The chemicals used in hydrometallurgy vary widely depending on the metal being extracted and the specific ore. Common agents include:
- Acids (such as sulfuric, hydrochloric, or nitric acid) for leaching base metals.
- Alkalis (like sodium hydroxide) for leaching aluminum and some uranium ores.
- Oxidizing agents (e.g., oxygen, chlorine) to dissolve metals like gold and platinum.
- Complexing agents (such as cyanide for gold) to stabilize the metal ions in solution.
Pyrometallurgy Overview
Definition and Process
Pyrometallurgy involves the extraction of metals from ores through high-temperature processes. This method is characterized by its efficiency in processing ores with a high metal content. The primary processes in pyrometallurgy are:
- Roasting: Heating ores in the presence of oxygen to remove volatile impurities and prepare them for smelting.
- Smelting: The process of melting ores to separate the metal from its ore.
- Refining: Purification of the extracted metal to achieve the desired level of purity.
High-temperature Techniques and Reactions
Pyrometallurgical techniques rely on thermal energy to initiate chemical reactions between the ore and a reducing agent or flux. Techniques include:
- Direct smelting, where the ore is directly exposed to high temperatures.
- Indirect smelting, using a blast furnace or flash smelting to efficiently process the ore.
Process Stages
Initial Stages in Hydrometallurgy
Leaching
Leaching is the first step in the hydrometallurgical process, involving the dissolution of metals into an aqueous solution. This process can be carried out in various ways:
- Heap leaching: Involves piling up crushed ore and applying the leaching solution to the top, allowing it to percolate through and dissolve the metals.
- Tank leaching: Ore is placed in large tanks where the leaching solution is added, and the mixture is agitated to enhance metal dissolution.
Solution Concentration and Purification
After leaching, the solution is concentrated and purified to remove impurities. This stage may involve:
- Precipitation: Adding agents to cause the metal to precipitate out of solution.
- Solvent extraction: Using organic solvents to selectively extract the desired metal.
- Ion exchange: Passing the solution through resins to replace unwanted ions with desirable metal ions.
Initial Stages in Pyrometallurgy
Roasting
Roasting involves heating the ore in the presence of oxygen to remove sulfur, carbon, and other impurities. The process can be adjusted to either partially or fully oxidize the ore, depending on the desired outcome.
Smelting
Smelting is a critical step in pyrometallurgy, requiring the melting of ores at high temperatures to separate the metal. Key aspects include:
- Flux addition: To reduce the melting point of the ore and assist in the removal of impurities.
- Reducing environment: Often necessary to reduce the metal oxides to pure metal.
Techniques and Technologies
Hydrometallurgical Techniques
Electrolytic Refining
Electrolytic refining involves the use of an electrical current to reduce dissolved metal ions and deposit pure metal onto a cathode. This technique is pivotal for refining metals like copper and nickel to high purity levels.
Solvent Extraction
Solvent extraction is a selective separation process in which a solvent is used to extract the desired metal from the leach solution. This method is particularly effective for rare earth elements and precious metals.
Pyrometallurgical Techniques
Flash Smelting
Flash smelting is an advanced smelting technique that combines the roasting and smelting processes. It involves blowing finely powdered ore concentrate with oxygen into a furnace, where it is instantly smelted.
Blast Furnace Operation
The blast furnace is a traditional method for extracting iron. It operates on the principle of chemical reduction wherein iron oxide is reduced to iron using coke as both a fuel and reducing agent. Blast furnaces are also used for smelting lead and copper.
Applications and Metals
Hydrometallurgy Applications
Precious Metals Extraction
Hydrometallurgy plays a crucial role in the extraction of precious metals like gold, silver, and platinum. The process involves:
- Cyanidation for gold and silver, using cyanide to dissolve these metals from their ores. This method is favored for its high efficiency and ability to process low-grade ores.
- Aqua regia for platinum group metals, utilizing a mixture of nitric and hydrochloric acid to dissolve and separate them from other elements.
Rare Earth Elements Recovery
The recovery of rare earth elements (REEs) is another significant application of hydrometallurgy, essential for electronics, magnets, and various high-tech applications. The process typically involves:
- Leaching of the ores with acid to extract REEs.
- Solvent extraction to separate and purify the individual REEs from the leachate.
Pyrometallurgy Applications
Iron and Steel Production
Pyrometallurgy is foundational to the iron and steel industry, with the blast furnace process being central to iron extraction:
- Iron production involves reducing iron ore in a blast furnace to produce pig iron, the primary ingredient in steel making.
- Steel production further refines pig iron, removing impurities and adjusting carbon content to produce various grades of steel.
Copper and Nickel Smelting
For copper and nickel, pyrometallurgy is a primary method of extraction:
- Copper smelting involves roasting copper sulfide ores, followed by smelting to produce copper matte, and finally, electrolytic refining to produce pure copper.
- Nickel smelting also utilizes high-temperature furnaces to extract nickel from its ores, often through a process similar to that of copper.
Environmental Impact
Hydrometallurgy and Sustainability
Waste Management Strategies
Hydrometallurgy offers effective waste management strategies, such as:
- Recycling of leaching agents to minimize waste.
- Tailings management to safely dispose of or repurpose the residue left after metal extraction.
Reduced Environmental Footprint
The environmental footprint of hydrometallurgy is generally
lower than that of pyrometallurgy due to:
- Lower energy consumption and greenhouse gas emissions, as many processes occur at ambient or slightly elevated temperatures.
- Minimal air pollution, as it avoids the high-temperature combustion processes that release significant pollutants.
Pyrometallurgy and the Environment
Emissions and Pollution Control
Pyrometallurgy’s environmental challenges include managing:
- Air emissions, including sulfur dioxide and carbon monoxide, which are mitigated through scrubbers and gas cleaning technologies.
- Solid waste, such as slag, managed through recycling and reuse in construction materials.
Energy Consumption
Pyrometallurgical processes are energy-intensive, requiring significant amounts of heat:
- This leads to higher carbon footprints compared to hydrometallurgical processes.
- Innovations in energy recovery and efficiency are critical to reducing the environmental impact.
Cost and Efficiency
Economic Aspects of Hydrometallurgy
Operating Costs
Hydrometallurgy can be less costly in operation due to:
- Lower energy requirements, significantly reducing fuel costs.
- The ability to process low-grade ores, opening up resources that are not economically viable for pyrometallurgy.
Efficiency and Scalability
Hydrometallurgy is noted for its:
- High efficiency in extracting metals from complex ores.
- Scalability, allowing for the processing of large volumes of ore with relatively small equipment footprints.
Economic Aspects of Pyrometallurgy
Capital Investment
Pyrometallurgy requires significant capital investment due to:
- The need for large-scale furnaces and energy infrastructure.
- Stringent environmental controls to mitigate pollution.
Energy Efficiency and Output
Despite its high energy consumption, pyrometallurgy offers:
- High throughput, making it suitable for processing large quantities of high-grade ores.
- Potentially lower per-unit costs at scale, benefiting from economies of scale.
Advancements and Innovations
Future of Hydrometallurgy
Technological Advancements
The future of hydrometallurgy looks promising with:
- Developments in leaching agents that are more effective and environmentally friendly.
- Innovations in separation technologies, such as advanced solvent extraction techniques and ion exchange resins, improving efficiency and selectivity.
Improvements in Efficiency and Sustainability
Efforts are ongoing to:
- Reduce the environmental impact through closed-loop systems and waste recycling.
- Enhance the economic viability of extracting lower-grade ores and recovering metals from electronic waste.
Future of Pyrometallurgy
Innovations in High-temperature Processes
Pyrometallurgy is evolving with:
- Advancements in furnace technology, such as flash smelting and top-submerged lance (TSL) smelting, reducing energy consumption and increasing process efficiency.
- Automation and process control improvements, enhancing consistency and reducing labor costs.
Environmental Mitigation Technologies
Significant research is focused on:
- Reducing emissions through better gas cleaning and sulfur capture technologies.
- Energy recovery systems that convert waste heat into electricity, offsetting some of the energy demands of the processes.
Frequently Asked Questions
What is hydrometallurgy?
Hydrometallurgy is a chemical metallurgical process used to extract and refine metals from their ores using aqueous solutions. This method is particularly effective for precious metals like gold and silver and metals used in electronics, such as copper and nickel. It offers a lower environmental impact and can efficiently process low-grade ores, making it a sustainable choice for metal extraction.
How does pyrometallurgy work?
Pyrometallurgy employs high temperatures to extract and purify metals from their ores. It involves steps like roasting, smelting, and refining, where ores are heated to extreme temperatures to facilitate the separation of metal from impurities. This method is highly efficient for processing ores with a high metal content and is commonly used in the extraction of iron, copper, and nickel.
Why choose hydrometallurgy over pyrometallurgy?
The choice between hydrometallurgy and pyrometallurgy depends on several factors, including the type of ore, environmental regulations, and cost considerations. Hydrometallurgy is often chosen for its ability to process low-grade ores with minimal environmental impact, making it a more sustainable option. Additionally, it requires less energy compared to pyrometallurgy, contributing to lower operational costs.
Can pyrometallurgy be environmentally friendly?
Improvements in technology and process efficiency have made pyrometallurgy more environmentally friendly than in the past. Innovations in pollution control, energy recovery, and waste management have significantly reduced the environmental impact of high-temperature metallurgical processes. While it still has a higher carbon footprint compared to hydrometallurgy, ongoing advancements aim to further mitigate its environmental effects.
Conclusion
The distinction between hydrometallurgy and pyrometallurgy is a fundamental aspect of metallurgical engineering, directly impacting the efficiency, sustainability, and environmental footprint of metal extraction and refining. These processes are not just technical choices; they reflect a balance between economic viability, environmental stewardship, and the demands of global markets for metals. As the world continues to seek more sustainable practices, the evolution of these metallurgical methods will play a critical role in shaping the future of the materials science industry.
The ongoing pursuit of advancements in both hydrometallurgy and pyrometallurgy underscores the industry’s commitment to innovation and environmental responsibility. By refining these processes, the field not only aims to increase efficiency and reduce costs but also to minimize the impact on the planet. The future of metal extraction and refining lies in the hands of these technologies, guiding the industry towards more sustainable and responsible metal production.