Enzymes play a pivotal role in the vast network of biochemical processes that sustain life. These biocatalysts accelerate chemical reactions, essential for everything from digestion to DNA repair. The focus of this discussion is on two types of enzymes, hydrolases and transferases, which differ fundamentally in their functions and the reactions they catalyze.
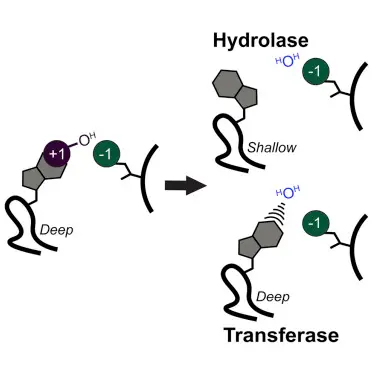
Hydrolases and transferases are classes of enzymes that mediate crucial biochemical transformations. Hydrolases catalyze the breaking of chemical bonds via the addition of water, a process known as hydrolysis. In contrast, transferases facilitate the transfer of functional groups from one molecule to another. These actions are critical in various biological pathways, influencing everything from energy production to the synthesis of complex molecules.
While both enzyme types are vital for life, their distinct mechanisms and roles underscore the complexity and specialization of cellular functions. Hydrolases are predominantly involved in catabolic processes that break down molecules, whereas transferases are crucial for anabolic processes, building complex molecules and modifying biochemical substrates to fulfill cellular needs.
Enzyme Basics
Definition of Enzymes
Enzymes are biological catalysts made primarily of proteins. They accelerate chemical reactions in the body without being consumed or permanently altered themselves. By reducing the energy barrier of reactions, enzymes ensure life-sustaining processes occur rapidly and efficiently.
Role in Metabolic Reactions
Enzymes play a central role in metabolic reactions, which are essential for the survival and functioning of cells. They help:
- Convert nutrients into energy
- Synthesize and break down cellular components
- Remove metabolic wastes
- Regulate metabolic pathways to respond to cellular environment changes
Common Characteristics
Despite their diverse functions, all enzymes share some common characteristics:
- Catalytic Property: They speed up reactions by providing an alternative pathway of lower activation energy.
- Specificity: Each enzyme is specific to a particular reaction or type of substrate.
- Sensitivity: Their activity can be influenced by environmental factors like temperature, pH, and the presence of inhibitors or activators.
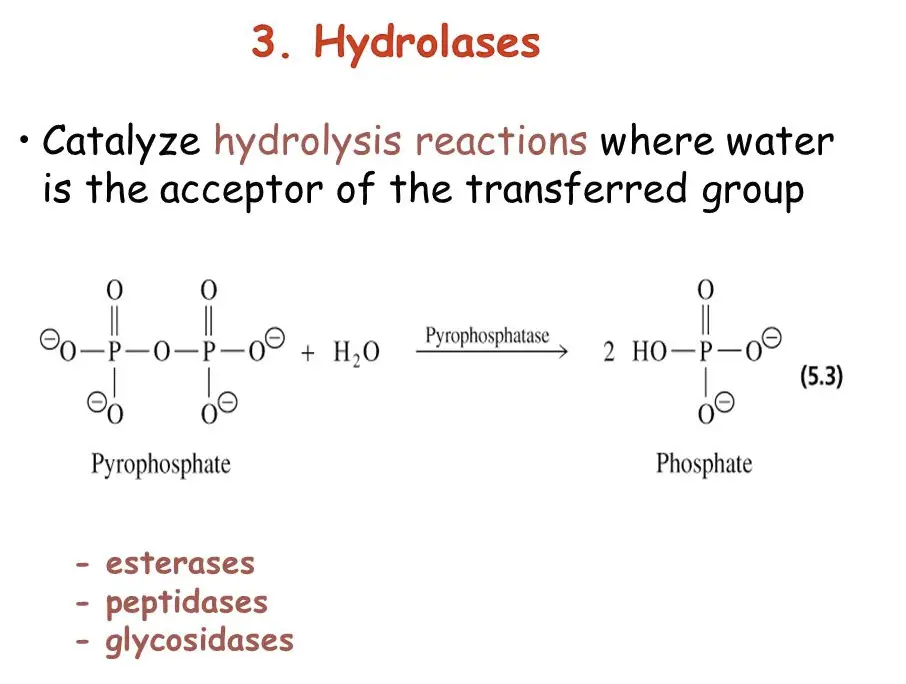
What is Hydrolase?
Definition and Function
Hydrolases are a class of enzymes that catalyze the hydrolysis of chemical bonds. They typically facilitate reactions where water molecules break bonds in other molecules, which is essential for processes like digestion and catabolism.
Types of Hydrolase Enzymes
Hydrolases can be classified based on the bond they target:
- Esterases: Break down ester bonds.
- Glycosidases: Split glycosidic bonds in carbohydrates.
- Peptidases: Cleave peptide bonds in proteins.
Examples in Biological Systems
Hydrolases are found in every cell and biological fluid. Examples include:
- Lipases: Break down fats in the digestive system.
- Amylase: Converts starch into sugars in the mouth and small intestine.
- DNAse: Degrades DNA molecules, playing a role in genetic regulation and waste management.
What is Transferase?
Definition and Function
Transferases are enzymes that catalyze the transfer of functional groups, such as a methyl or phosphate group, from one molecule to another. This activity is crucial for constructing complex molecules from simpler ones, modifying biochemical structures, and regulating biological pathways.
Types of Transferase Enzymes
Transferases are categorized by the type of group they transfer:
- Aminotransferases: Transfer amino groups.
- Kinases: Transfer phosphate groups, typically from ATP to another molecule.
- Methyltransferases: Move methyl groups to DNA, proteins, or other molecules.
Examples in Biological Systems
Transferases are integral in various biological functions:
- Transketolase: Critical for the pentose phosphate pathway, a metabolic pathway parallel to glycolysis.
- Glutathione S-transferase: Protects the cells by detoxifying harmful compounds.
- DNA methyltransferase: Modifies DNA, impacting gene expression and regulation.
Key Differences
Reaction Type
- Hydrolases: Break bonds by adding water.
- Transferases: Create new bonds by transferring functional groups.
Substrate Specificity
- Hydrolases: Act on a wide range of substrates, generally breaking them down.
- Transferases: Specifically modify substrates by adding groups that change the substrate’s function or reactivity.
Biological Roles
- Hydrolases: Mainly involved in degradative processes.
- Transferases: Primarily participate in biosynthetic processes, such as DNA replication and repair.
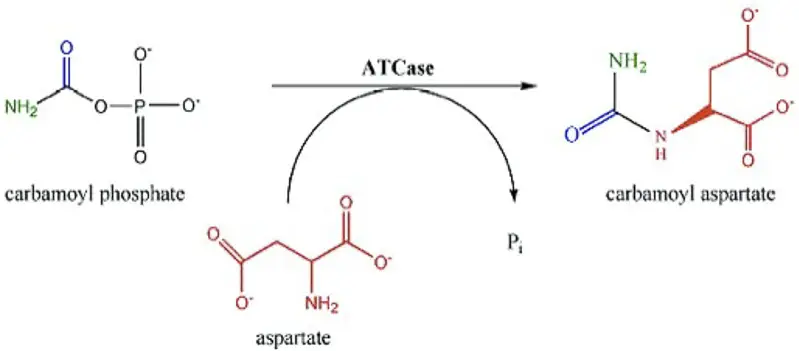
Reaction Mechanisms
Hydrolase Mechanism
General Process
Hydrolases bind to their substrate and cleave bonds by adding water, typically resulting in the formation of two smaller products. This reaction is crucial for breaking down large biomolecules into smaller, usable forms.
Example: Hydrolysis of Peptides
- Step 1: Peptidase binds to the peptide bond in a protein.
- Step 2: Water molecule is used to cleave the bond.
- Step 3: Amino acids are released, ready for further utilization in the body.
Transferase Mechanism
General Process
Transferases catalyze the movement of a functional group from a donor molecule, often a high-energy carrier like ATP, to a specific substrate. This action modifies the substrate’s chemical structure and biological activity.
Example: Transference of Phosphate Group
- Step 1: Kinase binds ATP and the substrate molecule.
- Step 2: Phosphate group is transferred from ATP to the substrate.
- Step 3: Modified substrate now carries a phosphate, altering its function in cellular signaling or metabolism.
Biological Importance
Hydrolase Significance
Digestion and Waste Processing
Hydrolases are critical for breaking down biomolecules such as proteins, fats, and carbohydrates into smaller components that the body can use. This process occurs primarily in the digestive system, where enzymes like amylase, lipase, and proteases play a key role. Beyond digestion, hydrolases also facilitate the breakdown and recycling of cellular waste. This function is crucial in maintaining cellular health and preventing the accumulation of harmful substances.
Regulation of Metabolic Pathways
Hydrolases regulate metabolic pathways by modulating the availability of key molecules and substrates necessary for various biochemical reactions. By controlling the rate at which these substances are produced or degraded, hydrolases ensure that metabolic processes like glycolysis and lipid metabolism proceed at optimal rates to meet the energy and structural needs of the cell.
Transferase Significance
Signal Transduction
Transferases, particularly kinases, are pivotal in signal transduction, the process by which a cell responds to external signals. These enzymes help transmit signals from the cell surface to the interior, influencing activities such as cell division, growth, and death. For instance, protein kinases modify other proteins by adding phosphate groups, changing the protein’s activity, localization, and interaction with other molecules.
DNA Transcription and Repair
Transferases are also essential in DNA transcription and repair. Enzymes like DNA methyltransferases add methyl groups to the DNA molecule, influencing gene expression patterns without altering the DNA sequence. This epigenetic regulation is crucial for normal development and adaptation to environmental changes. Additionally, transferases play a role in repairing DNA damage, thus helping to maintain genetic stability and prevent diseases such as cancer.
Clinical Relevance
Hydrolase in Medicine
Disease Associations
Certain hydrolase deficiencies are linked to a range of metabolic disorders. For example, deficiencies in lactase lead to lactose intolerance, while insufficient levels of certain lysosomal hydrolases result in lysosomal storage diseases such as Gaucher’s disease and Tay-Sachs disease.
Therapeutic Targets
Due to their crucial roles in metabolism and disease, hydrolases are significant therapeutic targets. Enzyme replacement therapies have been developed to treat disorders arising from hydrolase deficiencies. These treatments involve supplementing patients with functional versions of the enzyme they lack, thereby reducing symptoms and improving quality of life.
Transferase in Medicine
Disease Associations
Alterations in transferase activities can lead to serious health issues, including metabolic syndromes and cancers. Overactive transferases, such as certain kinases, are often found in cancer cells, where they promote uncontrolled cell growth and survival.
Therapeutic Targets
As a result, transferases, especially kinases, have become primary targets in cancer therapy. Drugs designed to inhibit kinase activity, known as kinase inhibitors, have been developed and are used to treat various types of cancer effectively. These inhibitors block the action of overactive kinases, thereby slowing down or stopping the progression of the disease.
Research and Innovations
Recent Discoveries in Enzyme Research
Recent advancements in enzyme research have led to the discovery of novel enzymes and mechanisms that could potentially treat a wide range of diseases. For example, researchers have identified new DNA repair enzymes that could be targeted to improve cancer treatment outcomes. Similarly, studies on enzymes involved in lipid metabolism have opened up new possibilities for addressing obesity and related metabolic disorders.
Advances in Therapeutic Applications
The field of enzyme therapeutics has seen significant developments, particularly in the design of enzyme inhibitors and mimetics. Modern approaches such as structure-based drug design and biotechnological enhancements are being employed to create more effective and specific enzyme inhibitors. Additionally, the use of enzyme-based diagnostics is expanding, allowing for more precise disease detection and monitoring.
Frequently Asked Questions
What are enzymes?
Enzymes are proteins that act as catalysts in biochemical reactions, significantly speeding up the rate of virtually all of the chemical processes required by the body.
How do hydrolases function?
Hydrolases catalyze reactions that involve the cleavage of bonds by the addition of water. They are vital for processes such as digestion, where large nutrient molecules are broken down into smaller, absorbable pieces.
What role do transferases play in the body?
Transferases are responsible for transferring functional groups between molecules, which is essential for the synthesis of key biomolecules and the regulation of metabolic pathways.
Can enzyme deficiencies affect health?
Yes, deficiencies in specific enzymes can lead to metabolic disorders or diseases. For example, a lack of certain hydrolases can result in problems with waste elimination and nutrient absorption, while deficiencies in transferases can affect cellular growth and repair.
Are enzymes used in medicine?
Enzymes are increasingly used in medical treatments and diagnostics. For example, certain hydrolases are employed to break down blood clots, and transferases are studied for their potential in gene therapy and cancer treatment.
Conclusion
The distinction between hydrolase and transferase enzymes highlights the intricate design of cellular metabolism. Each enzyme type is tailored to specific tasks, ensuring efficient and controlled biochemical reactions essential for maintaining cellular health and function. This understanding not only enhances our comprehension of biological processes but also guides medical science in developing enzyme-based therapies and diagnostics.
Insights into the differences and functionalities of these enzymes continue to drive scientific discovery and therapeutic innovation. As research advances, the potential to harness these enzymes more effectively in treating diseases looks increasingly promising, offering hope for new solutions to longstanding medical challenges.