Chemical bonding, the core of understanding molecular architecture, stands on two pivotal concepts: hybridization and overlapping. These terms, often used interchangeably by those new to chemistry, hold distinct meanings and roles in the formation of molecules. By exploring these phenomena, one can unlock a deeper understanding of how atoms come together to form the vast array of substances that make up our world.
Hybridization refers to the process by which atomic orbitals mix to form new hybrid orbitals, which in turn affects the geometry and bonding properties of molecules. Overlapping, on the other hand, involves the direct overlap of atomic orbitals to form covalent bonds between atoms. While both are essential for bond formation, they serve different functions within the context of molecular structure and stability.
These concepts are not just academic; they have practical applications in fields ranging from pharmaceuticals to materials science. Understanding the difference between hybridization and overlapping is crucial for predicting the physical and chemical properties of molecules, designing new compounds with desired properties, and advancing our overall knowledge of chemical reactions and interactions.
Basics of Chemical Bonds
Atomic Orbitals
Atomic orbitals are fundamental to chemistry. They represent the regions in an atom where electrons are likely to be found. Each orbital has a unique shape and energy level, directly influencing how atoms interact and bond with each other. Understanding these orbitals is crucial for grasping the basics of chemical bonding.
Bond Formation
The formation of chemical bonds is a complex process involving the interaction of electrons from different atoms. At its core, bond formation is about achieving stability. Atoms bond to fill their outer electron shells, reaching a more stable, lower energy state. This can occur through the sharing of electrons (covalent bonds), the transfer of electrons (ionic bonds), or the pooling of electrons (metallic bonds).
Key Terms
- Electron cloud: A region around the nucleus of an atom where its electrons are likely to be found.
- Energy levels: Specific regions where electrons can exist within an atom, each with a distinct amount of energy.
What is Hybridization?
Concept Overview
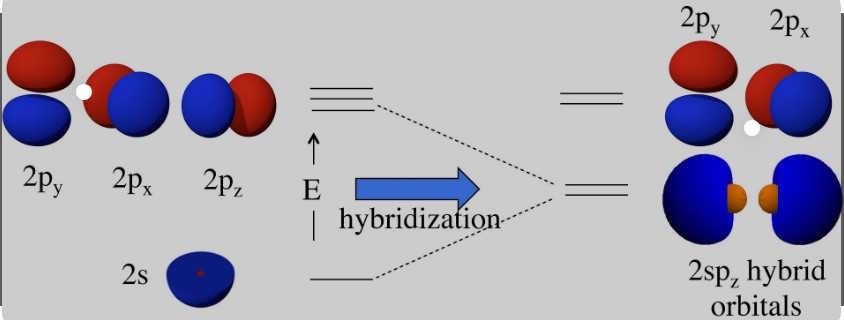
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form new, hybrid orbitals. This process is essential for explaining the shapes and bonding properties of molecules. Hybrid orbitals have different energy levels and geometries than the original atomic orbitals, enabling atoms to form bonds in specific orientations.
Types of Hybridization
There are several types of hybridization, each leading to different molecular geometries:
- sp hybridization: Forms two hybrid orbitals, leading to a linear shape.
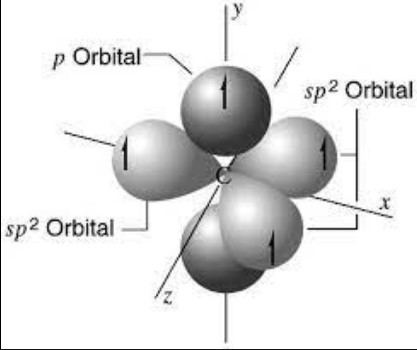
- sp2 hybridization: Creates three hybrid orbitals, resulting in a trigonal planar shape.
- sp3 hybridization: Produces four hybrid orbitals, giving a tetrahedral shape.
- sp3d and sp3d2 hybridization: These are involved in forming more complex shapes like trigonal bipyramidal (sp3d) and octahedral (sp3d2).
Mechanism
The process of hybridization involves several steps:
- Promotion of an electron to a higher energy level.
- Mixing of the atomic orbitals to form hybrid orbitals.
- Arrangement of these hybrid orbitals in space to minimize repulsion between electrons.
This reorganization allows for the formation of stable bonds with specific angles and distances between atoms.
What is Overlapping?
Concept Overview
Orbital overlapping is another critical concept in chemistry, essential for the formation of covalent bonds. It refers to the physical overlap of two atomic orbitals from different atoms, allowing for the sharing of electron pairs between those atoms. The strength and type of the bond formed depend on the extent and type of overlap.
Types of Overlapping
Orbital overlapping can result in two main types of covalent bonds:
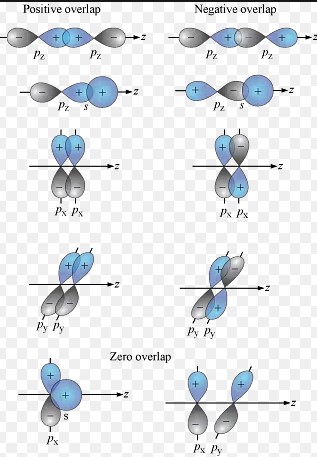
- Sigma (σ) bonds: Formed by the end-to-end overlap of orbitals. Sigma bonds are the strongest type of covalent bond and allow for free rotation around the bond axis.
- Pi (π) bonds: Result from the side-to-side overlap of orbitals. Pi bonds are weaker than sigma bonds and restrict the rotation of the bonded atoms, contributing to the rigidity of molecules.
Mechanism
The formation of a covalent bond through orbital overlapping involves:
- The approach of two atomic orbitals with compatible shapes and energy levels.
- Overlap of these orbitals to create a region of high electron density between the nuclei.
- Sharing of a pair of electrons in the overlapped region, leading to bond formation.
This mechanism is crucial for the construction of molecular structures and determines the strength and properties of the chemical bond.
Hybridization vs Overlapping
Fundamental Differences
The concepts of hybridization and overlapping in chemistry may seem similar at first glance, as both are crucial for bond formation. However, they represent different processes at the molecular level. Hybridization is the mixing of atomic orbitals within the same atom to form new, hybrid orbitals. This process is internal to an atom and is pivotal in determining the shape and bonding capabilities of molecules. Overlapping, conversely, involves the direct interaction between atomic orbitals from two different atoms, leading to the formation of covalent bonds.
Role in Chemical Bonding
- Hybridization shapes the way atoms bond by altering the orientation and types of orbitals available for bonding, which directly influences the geometry of molecules.
- Overlapping is the mechanism through which bonds are actually formed, by sharing electron pairs between atoms. The strength and characteristics of the bond—whether it’s a sigma or pi bond—depend on the type and extent of orbital overlap.
Visual Representation
Visual aids such as diagrams and models play a crucial role in illustrating the concepts of hybridization and overlapping. For example, diagrams showing sp3 hybridization illustrate how one s orbital and three p orbitals mix to form four equivalent sp3 orbitals, arranged in a tetrahedral geometry. Overlapping can be represented by showing how these hybrid orbitals overlap with orbitals from other atoms to form bonds, highlighting the electron density between the nuclei.
Applications in Chemistry
Hybridization in Molecules
Hybridization is observable in various complex molecules. For instance, the carbon atoms in ethylene (C2H4) undergo sp2 hybridization, resulting in a trigonal planar molecular shape. This allows for the formation of a pi bond in addition to single sigma bonds, contributing to the molecule’s unique properties.
Overlapping in Molecular Structures
The significance of overlapping is evident in the molecular structure determination. The double bond in oxygen (O2), for example, involves one sigma and one pi bond, the latter resulting from the side-to-side overlap of p orbitals. This overlapping not only dictates the bond length and strength but also influences the molecule’s reactivity and interaction with other molecules.
Factors Influencing
Impact on Molecular Properties
Both hybridization and overlapping have a profound impact on molecular properties. They affect physical properties like boiling and melting points, as well as chemical properties such as reactivity and polarity. For example, the difference in boiling points between ethane, ethene, and ethyne can be attributed to their different types of hybridization and resulting bond strengths.
Environmental Conditions
Environmental conditions such as temperature and pressure can also influence hybridization and overlapping. High temperatures, for instance, can provide the energy needed for the promotion of electrons, which can lead to different hybridization states. Similarly, pressure can affect orbital overlapping by altering the distance and orientation of molecules.
Common Misunderstandings
Hybridization
A common misconception about hybridization is that it alters the number of electrons in an atom. In reality, it involves the redistribution of the atom’s existing orbitals to form new, hybrid orbitals that are better suited for bonding.
Overlapping
Regarding overlapping, a frequent misunderstanding is that all overlapping orbitals contribute equally to bond strength. In reality, the type of overlap (end-to-end vs. side-to-side) and the orbitals involved (s, p, or hybrid) significantly affect the bond’s characteristics.
Importance in Modern Chemistry
Research and Development
Understanding hybridization and overlapping is vital in research and development, particularly in designing drugs and new materials. The ability to predict molecular shape and bonding interactions enables chemists to synthesize compounds with specific desired properties, such as increased efficacy or stability.
Educational Perspectives
From an educational standpoint, teaching the complexities of chemical bonding, including hybridization and overlapping, provides students with a foundational understanding of molecular chemistry. This knowledge is crucial for future scientists and engineers who will push the boundaries of what’s possible in chemistry and materials science.
FAQs
What is chemical bonding?
Chemical bonding refers to the force that holds atoms together in molecules or compounds. It results from the attraction between the atoms’ nuclei and electrons, leading to the formation of stable associations. These bonds can be ionic, covalent, metallic, or hydrogen bonds, each with unique properties and mechanisms.
How does hybridization affect molecular shape?
Hybridization significantly influences the molecular shape by determining the arrangement of the atom’s hybrid orbitals. For example, sp3 hybridization results in a tetrahedral geometry, sp2 in trigonal planar, and sp in linear. This rearrangement allows for an optimal spatial distribution of electrons, minimizing repulsion and stabilizing the molecule.
Why is orbital overlapping important in covalent bonds?
Orbital overlapping is crucial in the formation of covalent bonds as it facilitates the sharing of electron pairs between atoms. The extent of overlap between atomic orbitals determines the strength and length of the covalent bond. Sigma bonds result from end-to-end overlap and are stronger, while pi bonds arise from side-to-side overlap and provide additional stability to molecules.
Can a molecule undergo both hybridization and overlapping?
Yes, a molecule can undergo both hybridization and overlapping. Hybridization occurs first, forming hybrid orbitals with new shapes and orientations. These hybrid orbitals then overlap with those of other atoms to form covalent bonds. This two-step process is fundamental to the structure and stability of molecules.
Conclusion
The intricate dance between hybridization and overlapping forms the backbone of chemical bonding, shaping the endless diversity of molecular forms and functions. These concepts do more than just explain how atoms come together; they provide a framework for understanding the physical and chemical properties of substances, paving the way for innovations in chemistry and related fields.
Recognizing the distinct roles of hybridization and overlapping is not merely an academic exercise but a gateway to deeper insights into the molecular world. It is through this understanding that chemists continue to explore the boundaries of what is possible, crafting new materials and medicines that improve our lives.