Coordination chemistry is a fascinating field that explores the myriad ways in which metal atoms interact with surrounding ligands to form complex structures. These interactions not only dictate the structural configuration of these complexes but also determine their magnetic and electronic properties. At the heart of these properties lies the concept of spin states, a fundamental aspect that influences everything from a complex’s color to its reactivity and stability.
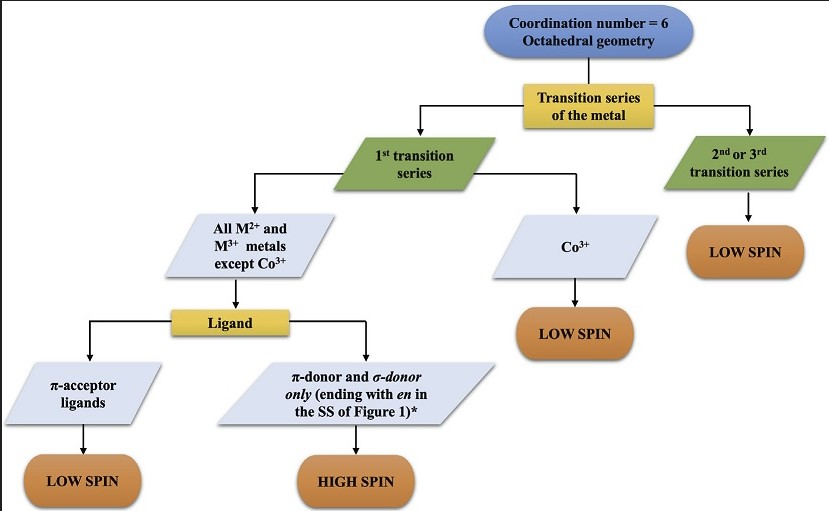
The difference between high spin and low spin complexes hinges on the electronic configuration of the metal center and how it interacts with its ligands. High spin complexes occur when the energy gap between the higher and lower energy d-orbitals is small, allowing electrons to occupy higher energy levels with parallel spins. Conversely, in low spin complexes, a larger energy gap encourages electrons to pair up in the lower energy orbitals, resulting in fewer unpaired electrons and a lower overall spin state.
Understanding these spin states is crucial for predicting the behavior and properties of coordination complexes. The distinction between high and low spin configurations affects not only the magnetic properties of these complexes but also their structural characteristics and their role in various chemical reactions and industrial processes. This aspect of coordination chemistry has significant implications in fields ranging from materials science to pharmaceuticals, highlighting the importance of spin states in modern chemistry.
Basics of Spin States
Definition of Spin States
In coordination chemistry, spin states refer to the possible orientations of an electron’s spin in a coordination complex. An electron can spin in two directions: up or down. When electrons in a complex have their spins aligned in the same direction, the complex is said to have a high spin state. Conversely, if electrons pair up with opposite spins, the complex adopts a low spin state. The total spin state of a complex significantly influences its magnetic and electronic properties.
Electron Pairing and Energy Levels
Electron pairing and energy levels are crucial in determining the spin state of a complex. Electrons inhabit atomic orbitals, and each orbital can hold two electrons with opposite spins. The energy levels of these orbitals can split under the influence of a ligand field, leading to different arrangements of the electrons and, subsequently, different spin states.
- High energy orbitals can accommodate unpaired electrons, leading to high spin states.
- Low energy orbitals encourage electrons to pair up, resulting in low spin states.
Role of the Ligand Field
The ligand field plays a pivotal role in determining the spin state of a complex. It describes the effect of ligands (molecules or ions surrounding the central atom) on the energy levels of d-orbitals in a metal ion. The strength of the ligand field influences whether a complex will favor a high or low spin state.
- Strong ligand fields cause a significant split in energy levels, favoring low spin states.
- Weak ligand fields result in a smaller energy gap, allowing for high spin states.
High Spin Complexes
Characteristics
Definition and Key Features
High spin complexes are coordination compounds where the energy gap between different sets of d-orbitals (split by the ligand field) is small enough that electrons occupy higher energy orbitals with parallel spins before pairing up in lower ones. Key features include:
- Multiple unpaired electrons
- High magnetic susceptibility
- Formed with weak field ligands
Types of Ligands Involved
Certain ligands, known as weak field ligands, are typically involved in the formation of high spin complexes. These include:
- Halides (Cl^-, Br^-, I^-)
- Phosphines (PR3)
- Alkyl groups
Formation
Ligand Field Theory Explanation
Ligand field theory explains the formation of high spin complexes by detailing how ligands interact with the d-orbitals of the central metal ion. In the presence of weak field ligands, the d-orbital splitting is minimal, allowing electrons to populate higher energy levels as unpaired, leading to a high spin state.
Energy Considerations
Energy considerations are central to the formation of high spin complexes:
- Low ligand field splitting energy allows electrons to occupy higher energy d-orbitals.
- The arrangement minimizes total energy by maximizing spin, despite higher orbital energy levels.
Examples
Common High Spin Complexes
Several common high spin complexes include:
- [��(�2�)6]2+[Fe(H2O)6]2+ – An iron complex with water as a ligand.
- [����4]2−[MnCl4]2− – A manganese complex with chloride ligands.
Industrial and Biological Relevance
High spin complexes find relevance in various industrial and biological contexts:
- Catalysis – Some high spin complexes are used as catalysts in organic reactions.
- Oxygen transport – Hemoglobin, though not always classified strictly under high spin complexes, demonstrates similar principles in its oxygen binding mechanism.
Low Spin Complexes
Characteristics
Definition and Distinguishing Traits
Low spin complexes form when the ligand field strength is strong enough to cause a significant energy gap between d-orbitals. This large gap encourages electrons to pair up in lower energy orbitals, leading to fewer unpaired electrons and a low spin state. Traits include:
- Fewer unpaired electrons
- Lower magnetic susceptibility
- Formed with strong field ligands
Ligand Types Favoring Low Spin
Strong field ligands that favor low spin complexes include:
- Cyanide (CN^-)
- Carbon monoxide (CO)
- Nitrosyl (NO)
Formation
Theoretical Background
The theoretical foundation for low spin complexes comes from ligand field theory. It postulates that strong field ligands cause a significant split in the d-orbitals of the central metal, making it energetically favorable for electrons to pair up in the lower energy orbitals.
Energy and Electron Configurations
For low spin complexes, the key energy and electron configurations considerations are:
- High ligand field splitting energy makes lower orbitals more energetically favorable for electron pairing.
- Electrons pair up, reducing the total spin and creating a low spin state.
Examples
Notable Low Spin Complexes
Examples of low spin complexes include:
- [��(��)6]4−[Fe(CN)6]4− – An iron complex with cyanide ligands.
- [��(��3)6]3+[Co(NH3)6]3+ – A cobalt complex with ammonia ligands.
Applications in Catalysis and Medicine
Low spin complexes have notable applications:
- Catalysis – They serve as important catalysts in industrial chemical reactions, such as hydrogenation processes.
- Medicine – Certain low spin complexes are used in medical imaging and as drugs for treating diseases like cancer.
Factors Influencing Spin States
Ligand Field Strength
The strength of the ligand field is a major factor in determining the spin state of a complex. Ligands that create a strong field around the central metal ion cause a larger splitting of the d-orbitals. This leads to low spin complexes as electrons prefer to pair up in the lower energy orbitals to minimize energy. On the other hand, ligands that generate a weaker field result in smaller d-orbital splitting, conducive to the formation of high spin complexes due to the accommodation of unpaired electrons in the higher energy orbitals.
- Strong field ligands like CN^- and CO lead to low spin complexes.
- Weak field ligands such as Cl^- and H_2O favor high spin complexes.
Metal Ion Characteristics
The characteristics of the metal ion, including its electronic configuration and oxidation state, significantly impact the spin state of a complex. Metal ions with a greater tendency to undergo pairing (due to lower energy differences between the orbitals) tend to form low spin complexes. Additionally, the size and charge of the metal ion influence the ligand field strength, thereby affecting the spin state.
- High oxidation states generally increase ligand field splitting, promoting low spin configurations.
- Electron configuration affects how easily electrons can be paired in the d-orbitals.
Geometric Considerations
The geometry of a complex plays a vital role in determining its spin state. Certain geometrical arrangements of ligands around the central metal ion can either enhance or diminish the ligand field strength, thus influencing the d-orbital splitting and the resultant spin state.
- Octahedral complexes with strong field ligands often result in low spin states.
- Tetrahedral or square planar complexes typically exhibit less d-orbital splitting, leading to high spin states, although there are exceptions based on ligand and metal ion characteristics.
Spin State Transition
Conditions for Transition
Spin state transitions occur under specific conditions that influence the energy balance between high and low spin states. These conditions include:
- Temperature changes can provide or remove the energy required for transitioning between spin states.
- Pressure variations and chemical modifications can also induce spin state transitions by altering the ligand field strength or the geometry of the complex.
Impact on Complex Properties
Transitioning between spin states can dramatically alter the properties of a complex. This includes changes in:
- Magnetic susceptibility: High to low spin transitions typically result in a decrease in magnetic susceptibility.
- Color: As the energy levels of the d-orbitals change, so does the absorption of light, leading to observable color changes in the complex.
Technological and Scientific Applications
Spin state transitions find applications in various fields:
- Sensors: Compounds that undergo visible color changes with spin transitions can be used as molecular sensors.
- Data storage: Materials that exhibit spin crossover properties are explored for their potential in high-density data storage devices.
Comparing High and Low Spin Complexes
Energy Levels and Stability
The stability and energy levels of high vs. low spin complexes differ significantly:
- Low spin complexes are generally more stable due to the lower energy configuration resulting from electron pairing.
- High spin complexes may possess higher energy levels because of the greater number of unpaired electrons but are less stable compared to their low spin counterparts.
Magnetic Properties
The magnetic properties of complexes depend largely on their spin states:
- High spin complexes exhibit greater magnetic susceptibility due to the presence of more unpaired electrons.
- Low spin complexes have reduced magnetic properties because of the lower number of unpaired electrons.
Color and Spectral Properties
The color and spectral properties of a complex are influenced by its spin state:
- High spin complexes often have different colors and spectral signatures compared to low spin complexes due to the difference in energy absorption and light emission patterns.
- Spin transitions can lead to notable changes in color, making such complexes useful in various applications including display technologies and sensors.
FAQs
What Determines High Spin vs. Low Spin Complexes?
The distinction between high and low spin complexes primarily depends on the ligand field strength, which influences the energy gap between the d-orbitals. Strong field ligands produce a larger energy gap, leading to low spin complexes, while weak field ligands result in a smaller gap, favoring high spin configurations. Metal ion characteristics and the geometric arrangement of the ligands also play crucial roles.
Why Are Spin States Important in Coordination Chemistry?
Spin states are vital in coordination chemistry because they influence the magnetic, electronic, and structural properties of complexes. These states affect a complex’s color, reactivity, and stability, impacting its applications in catalysis, magnetic materials, and medicine. Understanding spin states allows chemists to design complexes with desired properties for specific applications.
Can Spin States Change with Temperature?
Yes, the spin state of a complex can change with temperature. This phenomenon, known as spin crossover, occurs when the energy difference between high and low spin states is relatively small. Increasing or decreasing the temperature can provide enough energy to overcome this difference, causing a transition between spin states. This change can result in observable properties such as color change.
Conclusion
The exploration of high spin and low spin complexes opens up a window into the intricate world of coordination chemistry, shedding light on how subtle changes at the molecular level can lead to significant differences in physical and chemical properties. These concepts not only enrich our understanding of chemical bonding and reactivity but also pave the way for innovations across a broad spectrum of scientific and industrial fields.
As we continue to unravel the complexities of spin states, their impact on the development of new materials, catalysts, and therapeutic agents cannot be overstated. The future of coordination chemistry, therefore, lies in harnessing the potential of spin states to drive forward advancements in technology and medicine, making the study of high spin and low spin complexes a cornerstone of chemical research and application.
