Organic chemistry, a branch of chemistry concerned with the structure, properties, and reactions of organic compounds, plays a crucial role in the development of various industrial and pharmaceutical products. Within this field, the concepts of hemiacetals and hemiketals emerge as fundamental components, especially in the study of carbohydrates and their biochemical processes. These terms may seem intricate at first glance, but they hold the key to understanding many vital biological functions and chemical reactions.
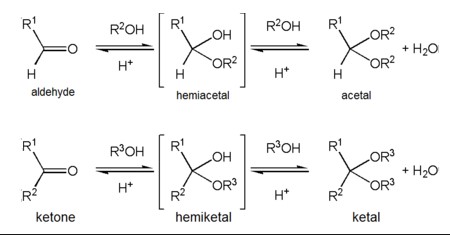
Hemiacetals and hemiketals are intermediates formed in the reaction pathway from aldehydes or ketones to acetals and ketals, respectively. Hemiacetals result from the addition of an alcohol to an aldehyde, while hemiketals form from the addition of an alcohol to a ketone. These reactions are reversible and play significant roles in the structure and behavior of sugars in biological systems.
The presence and transformation of these compounds are essential for the biochemical pathways that sustain life. They are not just steps in organic synthesis but pivotal in the structural formation of carbohydrates, affecting their reactivity and biological functions. This intricate dance between form and function in organic chemistry illuminates the profound complexity of biochemical processes and the elegant simplicity of nature’s design.
Basics of Organic Chemistry
Organic chemistry is the science of carbon-containing compounds, extending from simple molecules such as methane to complex structures like deoxyribonucleic acid (DNA). At its core, organic chemistry involves studying the structure, properties, composition, reactions, and synthesis of these compounds. This field is foundational for understanding life’s chemical basis and is pivotal in industries ranging from pharmaceuticals to materials science.
Organic Compounds Essentials
Organic compounds are defined by the presence of carbon atoms, linked together in chains or rings, and often bonded with hydrogen, oxygen, nitrogen, sulfur, and phosphorus. The versatility of carbon, with its ability to form four covalent bonds, allows for a vast array of organic compounds. This diversity is the backbone of countless biological processes and industrial applications.
Hydrocarbons are the simplest organic compounds, consisting solely of carbon and hydrogen. They serve as the foundation for more complex structures and are categorized into saturated (alkanes) and unsaturated (alkenes, alkynes) based on their carbon-carbon bonds.
Role of Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The presence and nature of a functional group can drastically alter a molecule’s properties and reactivity.
- Hydroxyl groups (-OH) make alcohols, contributing to solubility in water.
- Carbonyl groups (>C=O) define ketones and aldehydes and are key in energy-releasing reactions.
- Carboxyl groups (-COOH) are found in acids, playing a vital role in energy metabolism and protein construction.
Understanding these groups allows chemists to predict how organic molecules will interact and transform during chemical reactions.
Overview of Carbohydrates in Biochemistry
Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen, typically in a 1:2:1 ratio. They are crucial for life, serving as energy sources and structural components of cells. Carbohydrates are classified into three main types: monosaccharides (simple sugars), disaccharides (two monosaccharides linked together), and polysaccharides (long chains of monosaccharides).
- Glucose, a monosaccharide, is a primary energy source for cells.
- Sucrose, a disaccharide, is common table sugar.
- Cellulose and starch, polysaccharides, provide structural support in plants and energy storage, respectively.
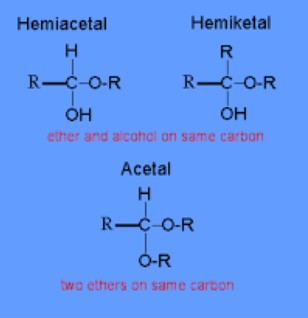
Hemiacetal Explained
Definition and Formation
A hemiacetal forms when an aldehyde reacts with an alcohol under acidic conditions. This reaction is a fundamental step in the biosynthesis of carbohydrates and other essential biomolecules. Hemiacetals can be recognized by their carbon atom bonded to an OH group and an OR group (where R represents an alkyl or aryl group), alongside hydrogen and another substituent.
Structure and Characteristics
The structure of hemiacetals includes a central carbon atom bonded to four different groups: a hydroxyl group (-OH), an alkoxyl group (-OR), a hydrogen atom, and another substituent, which can be a hydrogen atom (in simple hemiacetals) or another carbon-containing group. This diversity in bonding makes hemiacetals versatile intermediates in organic synthesis and metabolism.
Occurrence and Examples in Nature
Hemiacetals occur widely in nature, often as key intermediates in the formation of glycosidic bonds within carbohydrates. For example, during glycogen synthesis, glucose molecules are linked together through hemiacetal formations. Additionally, the cyclic forms of sugars, such as glucose and fructose, are hemiacetals, crucial for energy storage and transfer in biological systems.
Hemiketal Explained
Definition and Formation
Hemiketals are similar to hemiacetals but form from the reaction of a ketone with an alcohol, again under acidic conditions. This reaction is less common in simple laboratory synthesis but is pivotal in biological chemistry, particularly in the metabolism of ketoses, a type of sugar.
Structure and Characteristics
Hemiketals feature a central carbon atom connected to a hydroxyl group (-OH), an alkoxyl group (-OR), and two carbon-containing substituents. This configuration is crucial for the stability and reactivity of certain sugars and is a key intermediate in many biochemical transformations.
Occurrence and Examples in Nature
Like hemiacetals, hemiketals play a significant role in biochemistry. They are involved in the structure and function of ketoses, sugars that have a ketone group. Fructose, a common dietary sugar, can form a hemiketal in its cyclic form, impacting its sweetness and metabolic processing.
Key Differences
Structural Differences
The primary distinction between hemiacetals and hemiketals lies in their origin: hemiacetals derive from aldehydes, while hemiketals come from ketones. This difference affects their structure, with hemiacetals having one hydrogen atom attached to the central carbon, whereas hemiketals have two carbon-containing groups.
Formation Conditions
While both require acidic conditions for their formation, hemiacetals and hemiketals are formed through reactions with different types of carbonyl compounds (aldehydes for hemiacetals, ketones for hemiketals), affecting their prevalence and role in organic synthesis and metabolism.
Biological Significance
Both play crucial roles in biochemistry; however, their functions differ. Hemiacetals are vital in the formation and structure of carbohydrates, acting as intermediates in the synthesis of glycosidic bonds. Hemiketals, while less common, are essential in the metabolism of ketoses, influencing the processing and utilization of sugars in living organisms.
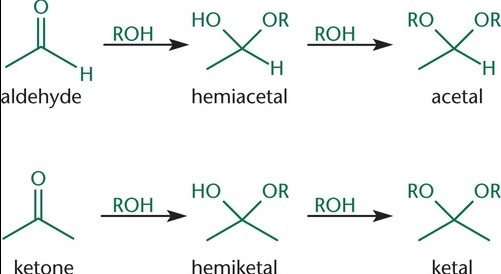
Formation Process
Step-by-step Formation of Hemiacetal
The formation of hemiacetals is a fundamental process in organic chemistry, involving a reaction between an aldehyde and an alcohol. Here’s how it unfolds:
- Aldehyde reacts with alcohol: The oxygen of the alcohol attacks the electrophilic carbon of the aldehyde.
- Proton transfer: An acid catalyst donates a proton (H⁺) to the oxygen of the hydroxyl group, increasing the molecule’s stability.
- Formation of hemiacetal: The result is a hemiacetal, characterized by a central carbon atom bonded to an -OH group, an -OR group, a hydrogen atom, and a substituent.
This process requires acidic conditions as a catalyst to proceed efficiently.
Step-by-step Formation of Hemiketal
Hemiketal formation mirrors that of hemiacetals, albeit starting from ketones instead of aldehydes:
- Ketone reacts with alcohol: The oxygen of the alcohol forms a bond with the carbonyl carbon of the ketone.
- Proton transfer: Similar to hemiacetal formation, an acid catalyst is crucial for donating a proton to the oxygen, stabilizing the intermediate.
- Formation of hemiketal: The final product is a hemiketal, with the central carbon bonded to an -OH group, an -OR group, and two carbon-containing substituents.
The process also necessitates acidic conditions for the reaction to occur smoothly.
Catalysts and Conditions
Both hemiacetal and hemiketal formations are catalyzed by acids. Common catalysts include:
- Sulfuric acid (H₂SO₄)
- Hydrochloric acid (HCl)
- P-Toluenesulfonic acid (PTSA)
The acidic environment is crucial for protonating the oxygen atom of the carbonyl group, making the carbon more electrophilic and facilitating the nucleophilic attack by the alcohol.
Biological Relevance
Role in Carbohydrate Chemistry
Hemiacetals and hemiketals are pivotal in the structure and function of carbohydrates. They are involved in:
- Formation of cyclic sugars: Many sugars exist in cyclic forms as hemiacetals or hemiketals, essential for their biological roles.
- Glycosidic bond formation: The reaction between hemiacetals/hemiketals and alcohols forms glycosidic bonds, linking sugar molecules in disaccharides and polysaccharides.
Importance in Metabolic Pathways
These compounds play significant roles in metabolic pathways:
- Energy release and storage: The conversion between hemiacetal and acetal forms of sugars regulates the availability of glucose for energy.
- Signal transduction: Modified sugars, often involving hemiacetal/hemiketal forms, are key in cellular signaling pathways.
Examples in Nature and Medicine
- Antibiotics: Some antibiotics function by mimicking the structure of hemiacetals, interfering with bacterial cell wall synthesis.
- Natural products: Hemiacetals and hemiketals are found in natural products with medicinal properties, such as certain antioxidants and anti-inflammatory agents.
Chemical Properties
Reactivity and Stability
Hemiacetals and hemiketals are relatively unstable and reactive:
- Equilibrium: They exist in equilibrium with their open-chain forms, making them easily convertible under certain conditions.
- Susceptibility to hydrolysis: Under acidic conditions, they can revert to their carbonyl and alcohol components.
Conversion to Other Compounds
Hemiacetals and hemiketals can undergo further reactions to form more stable compounds:
- Acetals and ketals: By reacting with another alcohol molecule, hemiacetals and hemiketals can form acetals and ketals, respectively, which are more stable and useful in protecting carbonyl groups during synthesis.
Role in Chemical Synthesis
Their reactivity makes hemiacetals and hemiketals useful intermediates in organic synthesis, including:
- Synthesis of glycosides: Important in producing various bioactive and medicinal compounds.
- Protecting groups: Serving as temporary modifications to protect sensitive functional groups during chemical reactions.
Applications and Examples
Industrial Applications
In the industrial context, the properties of hemiacetals and hemiketals find applications in:
- Food industry: Flavoring agents often involve hemiacetal and hemiketal structures for their aromatic properties.
- Polymer production: Some polymers are synthesized through reactions involving hemiacetal or hemiketal intermediates.
Use in Synthetic Chemistry
Their utility in synthesis is broad, including:
- Protecting groups: Protecting carbonyl groups during complex synthetic pathways.
- Synthesis of complex molecules: Serving as key intermediates in the synthesis of complex organic molecules, including natural products and pharmaceuticals.
Examples in Pharmaceuticals
- Drug formulation: Hemiacetal and hemiketal forms of drugs can influence their stability, solubility, and delivery mechanisms.
- Active pharmaceutical ingredients (APIs): Some APIs are designed to exploit the reactive nature of hemiacetals/hemiketals for targeted release in the body.
Frequently Asked Questions
What is a Hemiacetal?
A hemiacetal is a functional group formed when an alcohol adds to the carbonyl group of an aldehyde, creating a structure that contains both an ether and an alcohol. This reaction is crucial in organic synthesis and carbohydrate chemistry, providing a pathway for the formation of more complex molecules.
How is a Hemiketal Formed?
Hemiketals form through a similar process to hemiacetals, involving the addition of an alcohol to the carbonyl group of a ketone. This results in a compound that also contains both an ether and an alcohol group, playing a significant role in the chemistry of ketones and sugars.
Why are Hemiacetals and Hemiketals Important in Biology?
In biology, hemiacetals and hemiketals are vital for the structure and function of carbohydrates, including sugars. They are involved in the formation of glycosidic bonds in polysaccharides, which are essential for cell structure, energy storage, and communication within and between cells.
Can Hemiacetals Convert to Acetals?
Yes, hemiacetals can convert to acetals through a dehydration reaction, which involves the removal of water and the addition of a second alcohol molecule. This conversion is an important step in various organic synthesis processes, including the stabilization of carbohydrate molecules.
Conclusion
The exploration of hemiacetals and hemiketals uncovers the intricate connections between organic chemistry and biological systems. These compounds are not merely steps in chemical reactions but pivotal players in the fundamental processes that support life. Their study not only enhances our understanding of chemical reactions but also illuminates the complex mechanisms that underlie biological functions and the development of pharmaceuticals.
Understanding the differences and similarities between hemiacetals and hemiketals enriches our knowledge of organic chemistry and its applications in real-world scenarios. It empowers researchers and students alike to innovate and solve problems in the fields of medicine, biotechnology, and beyond, highlighting the indispensable nature of organic chemistry in advancing science and technology.