Titration is a fundamental technique in chemistry used to determine the concentration of an unknown solution. By adding a solution of known concentration, called the titrant, to the unknown solution until the reaction reaches a specific point, chemists can make precise measurements of chemical quantities. This process revolves around critical points known as the equivalence point and half equivalence point, each serving distinct roles in the titration curve.
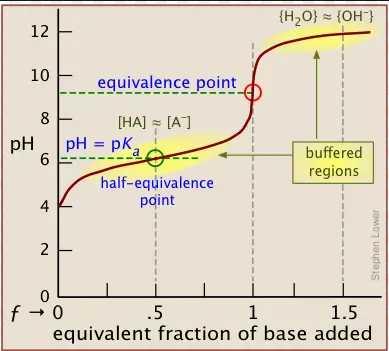
The equivalence point occurs when the amount of titrant added is just enough to completely react with the analyte in the solution, often resulting in a sharp change in some measurable property, like pH or electrical conductivity. On the other hand, the half equivalence point is reached when half of the analyte has reacted. It is particularly notable in pH titrations as it corresponds to the point where the pH equals the pKa of the acid being titrated, a crucial factor in understanding acid-base chemistry.
These points are not just academic curiosities; they are essential for the accurate determination of solution properties in various chemical, pharmaceutical, and biological processes. By examining their differences and applications, chemists can enhance their analytical precision and deepen their understanding of chemical reactions.
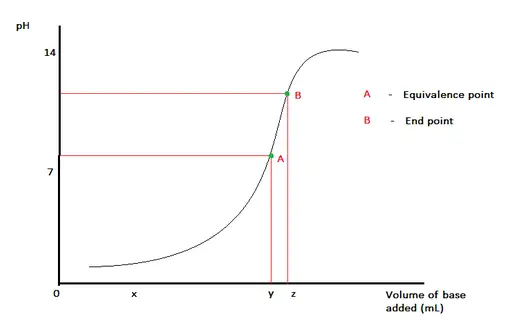
Equivalence Point Explained
Definition and Significance
The equivalence point in titration is a fundamental concept that marks the moment when the amount of titrant added to the analyte is exactly enough for the chemical reaction to complete. At this juncture, the added titrant and the analyte present in the solution are in stoichiometric balance, meaning the moles of titrant equal the moles of substance present in the sample.
This point is critical because it indicates that the chemical reaction has reached its completion. Understanding where the equivalence point lies in a titration curve allows chemists and scientists to accurately determine the concentration of an unknown solution. This is pivotal for applications across various fields such as environmental science, medicine, and manufacturing, where precise chemical measurements are necessary.
Role in Chemical Titration
In chemical titration, the role of the equivalence point is to provide a clear indicator for the endpoint of the reaction. It is often accompanied by a sudden change in a measurable property of the solution:
- pH level changes in acid-base titrations
- Precipitate formation in precipitation titrations
- Color change in complexometric titrations
The accurate identification of this point is essential for ensuring the reliability of the titration results. It directly influences the determination of the unknown concentration and the overall success of the titration process.
Half Equivalence Point Explained
Definition and Characteristics
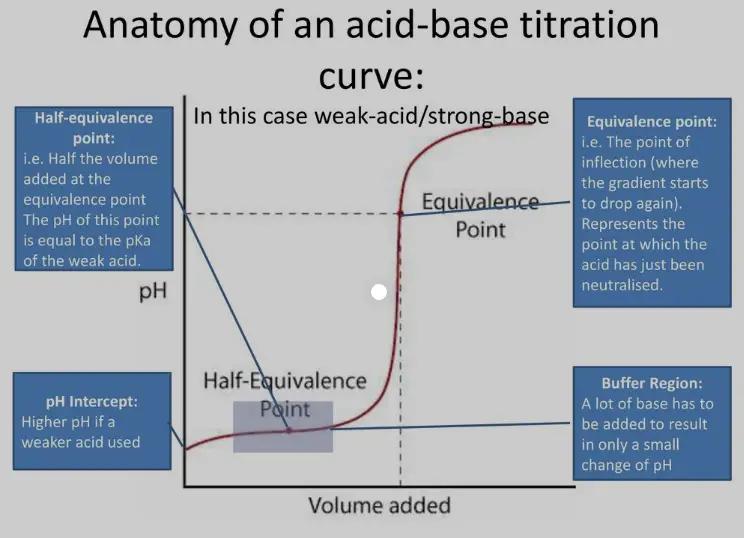
The half equivalence point occurs when half of the amount of the analyte has reacted with the titrant. At this point, exactly half of the original substance has been neutralized or reacted. This is particularly significant in pH titration because it corresponds to the pH level being equal to the pKa (acid dissociation constant) of the weak acid or base being titrated.
Importance in pH Titration
In pH titrations, the half equivalence point is used to determine the pKa of a weak acid or base, which is crucial for understanding its acid-base behavior in solution. Identifying the pKa allows chemists to:
- Predict buffering regions where the pH remains relatively stable despite additions of small amounts of acids or bases.
- Assess the strength of the acid or base.
- Optimize chemical synthesis and reactions by understanding the acid or base’s properties at different pH values.
Key Differences
Comparison of Definitions
While both points are milestones in a titration, the equivalence point represents the complete neutralization of the analyte, and the half equivalence point represents the stage where only half of the analyte has been neutralized. These points serve different purposes and are used to derive different sets of chemical information.
Impact on Titration Curve
The positions of these points on a titration curve are critical for interpreting the data:
- The equivalence point often results in a steep change in the curve, especially in strong acid-strong base titrations.
- The half equivalence point typically appears as a buffer region on the curve in weak acid-base titrations, showing less dramatic change.
Chemical Behavior at Each Point
The chemical behavior observed at these points can be quite distinct:
- At the equivalence point, the properties of the solution may change drastically, such as a sharp pH jump.
- At the half equivalence point, the pH of the solution equals the pKa, indicating a unique balance between the acid and its conjugate base, crucial for understanding buffering capacity.
Measurement Techniques
Tools and Indicators Used
To accurately identify the equivalence and half equivalence points, various tools and indicators are used:
- pH meters provide precise pH readings throughout the titration.
- Colorimetric indicators such as phenolphthalein or bromothymol blue change color at specific pH ranges.
- Conductivity meters measure changes in the solution’s ability to conduct electricity, which varies as ions react and form.
Procedures for Identifying Points
The procedure to identify these critical points involves careful measurement and observation:
- Prepare the solutions: Ensure that the analyte and the titrant are properly prepared and calibrated for the titration.
- Add the titrant gradually: Slowly add the titrant to the analyte, constantly stirring to mix thoroughly.
- Monitor the change: Use a pH meter or a conductivity meter to monitor changes in the solution. If using an indicator, watch for the color change.
- Record data: Note the volume at which significant changes occur, such as a sudden spike or plateau on the titration curve, indicating the equivalence or half equivalence point.
Practical Applications
Use in Academic Settings
The concepts of equivalence and half equivalence points are integral to the curriculum in both high school and college chemistry courses. Understanding these points helps students grasp fundamental principles in chemical reactions, particularly in acid-base and redox titrations. Here’s how they are applied in educational environments:
- Laboratory experiments: Students perform titrations to learn about stoichiometry, reaction kinetics, and chemical equilibrium.
- Analytical chemistry: These points are crucial for experiments involving the quantitative analysis of unknown substances, helping students develop precise analytical skills.
- Research projects: Advanced students might engage in research that involves detailed chemical analysis, relying on the accurate identification of these titration points to validate their findings.
Industrial Applications
In industry, the accurate determination of the equivalence and half equivalence points is critical for quality control and the development of various products. Industries such as pharmaceuticals, food and beverage, and environmental agencies rely on these measurements:
- Pharmaceuticals: Ensuring the correct dosage and chemical composition of drugs involves titration methods to maintain stringent regulatory standards.
- Food and beverage: Acidity levels in foods and drinks are often determined through titration, affecting flavor, preservation, and safety.
- Environmental monitoring: Testing water acidity and pollutant levels requires precise titration to assess compliance with environmental standards.
Common Challenges
Errors in Determination
Errors in determining the equivalence and half equivalence points can lead to significant discrepancies in data, affecting the outcome of experiments and industrial processes. Common sources of error include:
- Improper use of indicators: Choosing the wrong indicator can lead to incorrect identification of the point where the reaction has reached completion.
- Instrumental errors: pH meters and other measuring devices must be calibrated correctly; otherwise, readings may be inaccurate.
Mitigation Strategies
To overcome these challenges, several strategies can be implemented:
- Regular calibration of instruments: Ensure that all measuring devices are regularly checked and calibrated to provide accurate readings.
- Proper training: Chemists and technicians should be well-trained in titration techniques and the use of various indicators and instruments.
- Validation of methods: Regularly review and validate titration methods to ensure they remain suitable for the chemicals and reactions being analyzed.
Case Studies
Real-World Examples in Chemistry
Several case studies highlight the importance of accurately determining the equivalence and half equivalence points:
- Pharmaceutical formulation: A leading drug manufacturer uses titration to determine the precise concentration of active ingredients in its medications, ensuring each batch meets the required efficacy and safety standards.
- Acid rain analysis: Environmental scientists analyze samples from lakes affected by acid rain, using titration to measure the buffering capacity of the water and the impact of pollutants.
Analysis of Outcomes
The outcomes from these case studies often lead to improvements in product quality and environmental health:
- Improved drug safety and efficacy: By accurately measuring active ingredients, pharmaceutical companies can ensure that their products are both safe and effective for consumer use.
- Enhanced environmental protection: Understanding the chemical properties of natural waters helps in crafting more effective regulations and cleanup strategies to combat pollution.
Frequently Asked Questions
What is a titration curve?
A titration curve graphically represents the change in a measurable property of a solution, like pH or conductivity, as a titrant is added. It is crucial for identifying the equivalence and half equivalence points during a titration, providing visual insight into the chemical behavior of the solution throughout the titration process.
Why is the equivalence point important?
The equivalence point is crucial because it signifies the exact moment when the quantity of titrant added is stoichiometrically equivalent to the quantity of analyte in the solution. This point is essential for calculating the precise concentration of the unknown solution, making it fundamental in chemical analysis and various industrial applications.
How is the half equivalence point used in pH titration?
In pH titration, the half equivalence point is significant because it marks where the pH of the solution equals the pKa of the acid being titrated. This information is invaluable for determining the acid dissociation constant of a compound, which is critical for understanding and predicting the buffering capacity and pH stability of solutions.
Can equivalence points vary between different titrations?
Yes, the location of the equivalence point can vary depending on the type of titration and the substances involved. For example, in acid-base titrations, the equivalence point can occur at different pH values based on the strength and nature of the acids and bases involved. Similarly, in redox titrations, the point is determined by the specific oxidation and reduction reactions taking place.
Conclusion
The distinction between the half equivalence point and the equivalence point is more than a simple academic detail; it is a cornerstone of quantitative chemical analysis. By understanding these concepts, chemists can enhance their methodological accuracy and gain deeper insights into the chemical dynamics of reactions.
Through careful study and application of these principles, practitioners can optimize their experiments and ensure the reliability of their results in research, quality control, and beyond. As chemistry continues to evolve, so too will the techniques and technologies used to measure and exploit these critical points in titration.