Adsorption phenomena play a pivotal role in environmental engineering, catalysis, and various purification processes, marking their significance across a broad spectrum of scientific research and industrial applications. This process, where molecules of a gas or liquid adhere to the surface of a solid or a liquid, is fundamental to the development of efficient materials and processes in numerous fields. The precise understanding of how molecules interact with surfaces is crucial for optimizing these applications.
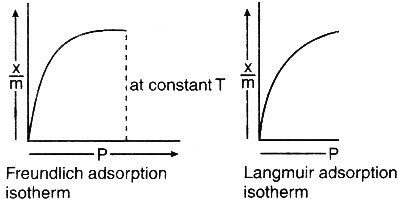
The difference between Freundlich and Langmuir adsorption isotherms lies primarily in their approach to modeling these interactions. The Freundlich isotherm describes adsorption on heterogeneous surfaces and assumes that the adsorption process is not limited to the formation of a single layer, while the Langmuir isotherm focuses on adsorption to homogenous surfaces, suggesting that adsorption occurs until a monolayer of adsorbate is formed on the adsorbent surface.
These two models provide frameworks for predicting how adsorbates will interact with various surfaces under different conditions. They help scientists and engineers design more effective adsorbent materials, understand adsorption processes at a molecular level, and develop applications ranging from water purification systems to the creation of highly efficient catalytic surfaces. The distinction between Freundlich and Langmuir adsorption isotherms reflects the diversity and complexity of adsorption phenomena, underscoring the need for multiple models to capture the nuances of these processes.
Adsorption Basics
What is Adsorption?
Adsorption is a surface phenomenon where molecules from a fluid phase (gas or liquid) accumulate on the solid or liquid surface, forming a thin layer. This process is essential in various applications, including water treatment, air purification, and catalysis. Adsorption relies on the interaction between the surface and the adsorbate molecules, leading to their accumulation. The efficiency and capacity of adsorption can significantly impact the design and functionality of materials and processes across many industries.
Adsorption vs. Absorption
While both adsorption and absorption involve the uptake of substances, they differ fundamentally in their processes. Adsorption occurs on the surface of a material, where molecules adhere to the exterior. In contrast, absorption involves the entire volume of the material, where substances are taken up by the volume and incorporated into the material’s matrix. Understanding this distinction is crucial for selecting the right process in applications such as filtration, where the choice affects the design and operation of the system.
Freundlich Isotherm
Overview
The Freundlich isotherm, developed in 1906 by Herbert Freundlich, stands as one of the earliest attempts to model the adsorption process. It was originally formulated to describe the adsorption equilibrium of solutes from a liquid solution, but has since been applied to gases as well. The isotherm is empirical, meaning it is based on experimental observations rather than derived from first principles.
Key Features
The Freundlich isotherm is particularly noted for its flexibility in modeling adsorption on heterogeneous surfaces. It suggests that the adsorption capacity of a surface for a specific adsorbate:
- Varies with concentration
- Is not restricted to a single layer
This model is empirical, making it adaptable to a wide range of conditions without needing detailed knowledge of the surface’s physical properties.
Mathematical Model
The Freundlich equation is expressed as: �=���1/�q=KfC1/n where:
- �q is the amount of adsorbate adsorbed per unit mass of adsorbent,
- �C is the concentration of adsorbate,
- ��Kf and 1/�1/n are constants indicating the adsorption capacity and intensity, respectively.
This equation showcases the non-linear relationship between the concentration of adsorbate and the amount adsorbed, reflecting the complexity of real-world adsorption processes.
Applications
The Freundlich isotherm finds applications in:
- Environmental engineering, particularly in the adsorption of contaminants from water.
- Agriculture, in understanding soil nutrient retention.
- Catalysis, for surface reaction studies.
Its versatility makes it a valuable tool in the preliminary assessment of adsorption systems.
Langmuir Isotherm
Overview
The Langmuir isotherm, developed by Irving Langmuir in 1918, provides a theoretical model for adsorption that assumes a homogeneous surface with fixed adsorption sites. This model posits that once a site is occupied, no further adsorption can occur at that site, leading to a finite number of adsorbate layers—typically one monolayer.
Key Features
The Langmuir isotherm is characterized by its simplistic assumptions:
- Homogeneous adsorption sites
- No interaction between adsorbed molecules
- Monolayer adsorption
These features make the Langmuir model particularly suited for systems where surface uniformity and single-layer adsorption are expected.
Mathematical Model
The Langmuir equation is given by: 1�=1����+1�������q1=qmax1+KLqmaxC1 where:
- �q is the amount adsorbed per unit mass of adsorbent,
- �C is the equilibrium concentration of the adsorbate,
- ����qmax is the maximum adsorption capacity,
- ��KL is the Langmuir constant related to the affinity of the binding sites.
This model introduces the concept of a saturation point, beyond which no additional adsorption occurs.
Applications
The Langmuir isotherm is widely used in:
- Chemical engineering for designing adsorption systems.
- Pharmaceuticals in drug delivery mechanisms.
- Material science for the development of adsorbent materials with specific characteristics.
It serves as a critical tool for the analysis and design of systems where monolayer adsorption on homogeneous surfaces is a key process.
Comparing Freundlich and Langmuir
Surface Homogeneity
Freundlich Isotherm Assumptions
The Freundlich isotherm assumes heterogeneous surfaces. It considers that surfaces have a variety of adsorption sites with different energies for adsorption. This variance in surface energy leads to a non-uniform adsorption layer, reflecting the complexity and diversity of real-world surfaces.
Langmuir Isotherm Assumptions
In contrast, the Langmuir isotherm assumes that the adsorbing surface is homogeneous. Each adsorption site is identical, and each has the same affinity for the adsorbate molecules. This model simplifies the adsorption process to a uniform, single layer of molecules, allowing for straightforward mathematical modeling.
Adsorption Layers
Freundlich on Adsorption Layers
The Freundlich isotherm does not limit the formation of adsorbate layers. It effectively allows for multilayer adsorption, with the first layer of adsorbate potentially acting as a new adsorbent surface for subsequent layers. This flexibility is more aligned with complex adsorption scenarios where multiple layers can form.
Langmuir on Adsorption Layers
The Langmuir isotherm, however, posits that adsorption occurs in a monolayer. Once each site on the surface is occupied by an adsorbate molecule, no further adsorption can occur. This model is particularly relevant for processes where a controlled, uniform coverage of adsorbate is crucial.
Application Scope
Freundlich Isotherm Applications
The Freundlich isotherm, with its broad assumptions regarding surface heterogeneity and multilayer adsorption, is suited for complex systems where the surface characteristics and adsorption behaviors vary significantly. It is widely used in soil science, wastewater treatment, and heterogeneous catalysis where the adsorbent surfaces are not uniform.
Langmuir Isotherm Applications
The Langmuir isotherm’s assumptions make it ideal for applications involving homogeneous surfaces and where monolayer adsorption is desired or expected. This includes applications in gas masks, sensors, and certain catalytic processes. Its precise and predictable modeling facilitates the design and development of adsorption systems in chemical engineering and material science.
Mathematical Complexity
Freundlich Isotherm Complexity
The Freundlich isotherm, being empirical, is relatively straightforward to apply, as it directly relates to observed adsorption data without necessitating a detailed understanding of the adsorption mechanism. However, its empirical nature means it might not provide deep insights into the physics of the adsorption process.
Langmuir Isotherm Complexity
The Langmuir isotherm is more theoretically grounded, offering a clear, mechanistic understanding of adsorption. Its mathematical model, though more complex than Freundlich’s, provides valuable insights into the adsorption capacity and kinetics, making it a powerful tool for designing and analyzing adsorption systems with high precision.
Practical Implications
Choosing the Right Model
Selecting between the Freundlich and Langmuir isotherms depends on the specifics of the adsorption process and the characteristics of the adsorbent and adsorbate. Considerations include:
- Surface uniformity: Use Langmuir for homogeneous and Freundlich for heterogeneous surfaces.
- Adsorption layer: For monolayer adsorption, Langmuir is preferred; for multilayer or non-specific layer adsorption, Freundlich is more applicable.
- Desired accuracy: Langmuir provides more detailed insights for theoretical and practical applications where the adsorption process can be tightly controlled.
Real-world Applications
Water Treatment
In water treatment, Freundlich isotherms are often used to model the adsorption of pollutants on activated carbon, reflecting the heterogeneous nature of carbon surfaces. Meanwhile, Langmuir isotherms may be applied in designing filters for specific chemicals where a uniform adsorption layer ensures efficient removal.
Catalysis
In catalysis, Langmuir isotherms are crucial for understanding how gases adsorb onto metal surfaces, impacting the design of industrial catalysts for reactions like hydrogenation. The Freundlich isotherm finds its place in catalysis involving complex surfaces or when the catalyst’s surface properties vary significantly.
Material Science
Material science benefits from both isotherms for developing new adsorbent materials. Langmuir isotherms help in the design of nanomaterials with uniform surface properties for drug delivery systems. Freundlich isotherms are useful in developing porous materials where the adsorption behavior is not strictly uniform or monolayered.
Frequently Asked Questions
What is Adsorption?
Adsorption is the process by which atoms, ions, or molecules from a substance (it could be a gas, liquid, or dissolved solid) adhere to a surface of another substance. This phenomenon is crucial for various applications, including air and water purification, in catalysis, and in the medical field for drug delivery systems.
How Do Freundlich and Langmuir Isotherms Differ?
The main difference between Freundlich and Langmuir adsorption isotherms is in their assumptions and applications. The Freundlich isotherm assumes heterogeneous surface adsorption without a limit to the layers of adsorption, making it ideal for complex systems. The Langmuir isotherm, on the other hand, models adsorption to homogeneous surfaces with adsorption occurring until a monolayer is formed, suitable for more simplified systems.
Why Are Adsorption Isotherms Important?
Adsorption isotherms are crucial because they provide a theoretical framework that helps understand and predict how adsorbates will interact with adsorbent surfaces. This knowledge is essential for designing and optimizing industrial processes, such as purification, separation techniques, and in the synthesis of materials with specific properties.
Can Freundlich and Langmuir Isotherms Apply to All Materials?
No, Freundlich and Langmuir isotherms cannot apply to all materials universally. The Freundlich isotherm is more suited to heterogeneous surfaces and multi-layer adsorption, while the Langmuir isotherm is ideal for homogeneous surfaces where adsorption forms a monolayer. The choice between them depends on the specific characteristics of the adsorbent and adsorbate.
Conclusion
The understanding of Freundlich and Langmuir adsorption isotherms illuminates the complex nature of adsorption processes, providing essential insights that drive innovation in materials science, environmental engineering, and many other fields. By distinguishing between these two models, researchers can tailor adsorption-based solutions to a wide range of applications, enhancing the efficiency of processes from industrial catalysis to pollution control.
Choosing the appropriate isotherm model is more than an academic exercise; it is a practical necessity that impacts the development of technologies and processes across numerous industries. As our understanding of adsorption deepens, the refinement of these models and the introduction of new ones will continue to play a critical role in advancing science and engineering to meet the challenges of the future.