Proteins are fundamental to life, serving as the building blocks for cells and performing a vast array of functions essential for biological processes. These macromolecules are made up of amino acids, whose sequences determine the three-dimensional structures proteins assume. The formation of these structures, known as protein folding, is critical because it directly influences the protein’s functionality and stability within the body.
The difference between folded and unfolded proteins lies in their structural configurations. Folded proteins have a defined three-dimensional shape crucial for their specific functions, such as catalyzing biochemical reactions or transporting molecules. In contrast, unfolded proteins lack a stable structure, often leading to non-functional or aggregative states that can disrupt cellular function.
Understanding the states of protein configurations is not just an academic pursuit but a practical one as well, influencing everything from genetic research to disease treatment. The behavior of proteins, whether folded or unfolded, plays a significant role in health and disease, making their study a focal point in biochemistry and molecular biology.
Protein Basics
Definition and Roles
Proteins are complex, organic compounds composed of amino acids that are crucial to the functioning of all living cells. They serve a multitude of roles within biological systems, including acting as enzymes, structural elements, signaling molecules, and transport carriers. For example, enzymes, a type of protein, catalyze biochemical reactions essential for digestion and metabolism. Structural proteins, like collagen, provide support in connective tissues and bones. Proteins are indispensable in nearly every process within a cell, from the replication of DNA to the transport of molecules across cell membranes.
Structure Types
Proteins can be broadly classified into three structure types based on their shape and solubility: fibrous, globular, and membrane proteins.
- Fibrous Proteins: These are long, insoluble, and structural. They provide support and strength to cells and tissues. Examples include keratin in hair and nails, and collagen in connective tissues.
- Globular Proteins: These are compact, soluble, and generally spherical in shape. They perform a variety of functions, including catalyzing metabolic reactions, transporting small molecules, and regulating biological processes. Hemoglobin, which transports oxygen in the blood, is a globular protein.
- Membrane Proteins: Embedded in the lipid bilayers of cells, these proteins act as gatekeepers, controlling what enters and exits the cell. They are crucial for communication between the internal and external environments of the cell.
Protein Folding
What is Folding?
Protein folding is the physical process by which a protein chain acquires its native 3-dimensional structure, a conformation that is biologically functional. This folding is determined by the genetic sequence of the protein, and the correct structure is essential for the protein’s specific function.
Process and Mechanisms
The process of protein folding is an intricate sequence of events guided by the protein’s amino acid sequence and influenced by the cellular environment. The steps typically include:
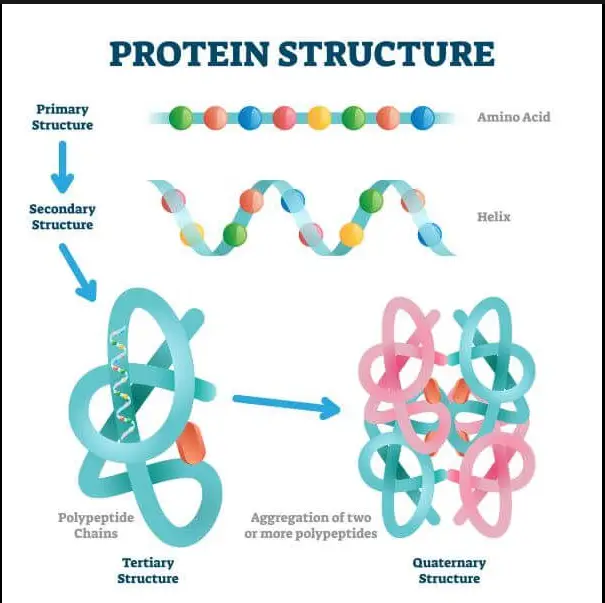
- Primary Structure: The sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local structures that form within the polypeptide due to interactions between atoms in the backbone. These include alpha helices and beta sheets.
- Tertiary Structure: The overall three-dimensional structure of a single protein molecule; the spatial arrangement of secondary structures.
- Quaternary Structure: The structure formed by several protein molecules (polypeptide chains), usually called protein subunits in this context, which function as a single protein complex.
Chaperone proteins assist in the folding process, ensuring the protein folds into the correct structure and helping to refold proteins that have misfolded.
Unfolded Proteins
Characteristics
Unfolded proteins are those that have not yet achieved or have lost their functional three-dimensional structure. These proteins do not perform their specific biological functions and can aggregate, leading to cellular stress and toxicity.
Role in Cells
The role of unfolded proteins is dual. Under normal conditions, they can be precursor states in the folding process, waiting to assume their functional conformation. However, when misfolding occurs, these proteins can accumulate and lead to the development of various diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s disease, which are characterized by the presence of protein aggregates in cells.
Folding Errors
Causes and Consequences
Protein misfolding can result from a variety of factors, ranging from genetic mutations to environmental stressors. Errors in the amino acid sequence, improper post-translational modifications, or stressful cellular conditions such as high temperatures or pH imbalances can lead to incorrect folding. The consequences of these misfolded proteins are severe, including loss of function and formation of toxic aggregates that can disrupt cellular processes and lead to cell death.
Diseases Linked to Misfolding
Protein misfolding is directly linked to a range of diseases, often referred to as proteinopathies or conformational diseases. These include:
- Alzheimer’s Disease: Characterized by the accumulation of beta-amyloid plaques and tau protein tangles in the brain.
- Parkinson’s Disease: Marked by the buildup of alpha-synuclein fibrils in neurons.
- Huntington’s Disease: Caused by the aggregation of huntingtin protein with expanded polyglutamine sequences.
- Cystic Fibrosis: Resulting from a misfolded CFTR protein that fails to regulate chloride and water transport in cells.
Each of these conditions highlights how crucial proper protein folding is for maintaining cellular health and overall organismal function.
Protein Folding Studies
Techniques and Methods
The study of protein folding has evolved significantly, utilizing sophisticated techniques to understand and manipulate these processes:
- X-ray Crystallography: Allows scientists to determine the atomic structure of protein crystals, providing insights into the folded structures.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Used for studying proteins in solution, offering a view of protein dynamics and interactions.
- Cryo-electron Microscopy (cryo-EM): Enables the visualization of proteins in their native state without the need for crystallization.
- Molecular Dynamics Simulations: Computational methods that simulate the physical movements of atoms and molecules, predicting folding pathways and structures.
Key Discoveries and Impacts
Significant discoveries in protein folding include the realization that the amino acid sequence of a protein determines its 3D structure, as proposed by Christian Anfinsen, earning him a Nobel Prize. More recently, the development of the Protein folding AI by Google’s DeepMind, which can accurately predict protein structures, marks a pivotal advancement in the field.
Applications
Biotechnology and Medicine
Protein folding research has profound implications in biotechnology and medicine, influencing drug design, genetic engineering, and therapeutic strategies:
- Drug Design: Understanding protein structures allows for the creation of drugs that can specifically target and bind to these molecules, enhancing efficacy and reducing side effects.
- Gene Therapy: Techniques that correct genetic mutations causing misfolding can potentially cure genetic diseases.
- Therapeutic Proteins: Designing and producing proteins with therapeutic functions, such as enzymes in enzyme replacement therapy for diseases like Gaucher’s disease.
Future Research Directions
Looking forward, the field of protein folding is poised to tackle some of the most challenging questions in science and medicine. Future research directions include:
- Improving Prediction Models: Enhancing the accuracy and efficiency of computational models to predict protein folding and interactions.
- Understanding Intrinsically Disordered Proteins: These are proteins that lack a stable structure yet are crucial for cellular functions, and their study could revolutionize our understanding of cellular machinery.
- Targeting Misfolding Diseases: Developing more effective treatments that can prevent or reverse protein misfolding and aggregation in diseases.
Frequently Asked Questions
What is Protein Folding?
Protein folding is the process by which a protein structure assumes its functional, three-dimensional form from a random coil. This folding is guided by the amino acid sequence of the protein and is crucial for biological function.
Why Do Proteins Unfold?
Proteins may unfold due to changes in their environment such as pH, temperature, or chemical exposure. Unfolding can also result from mutations in the amino acid sequence which disrupt the normal folding process.
How Are Misfolded Proteins Corrected?
Cells utilize molecular chaperones to assist in the refolding of misfolded proteins. If refolding fails, such proteins are often degraded by the cell to prevent accumulation and potential toxicity.
What Are Diseases Related to Protein Misfolding?
Many diseases, such as Alzheimer’s, Parkinson’s, and cystic fibrosis, are associated with protein misfolding. Misfolded proteins can form aggregates that disrupt cellular function and cause disease.
How is Protein Folding Studied?
Researchers use techniques like X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy to study protein structures and understand folding mechanisms.
Conclusion
The intricate dance between folded and unfolded proteins is a cornerstone of cellular function and health. A clear understanding of this balance helps illuminate the pathogenesis of numerous diseases and underscores the importance of protein studies in medical science.
Advancements in research continue to reveal the complexities of protein behavior, fostering innovations in drug design and therapeutic interventions. Recognizing the pivotal role that protein folding plays in health and disease not only expands our scientific knowledge but also paves the way for future breakthroughs in biotechnology and medicine.

