Apatite minerals, specifically fluorapatite and hydroxyapatite, are crucial in various scientific and industrial applications, yet they often go unrecognized outside specialized fields. Fluorapatite, rich in fluorine, and hydroxyapatite, predominantly containing hydroxyl groups, both belong to the apatite group of phosphates, which have diverse uses from agriculture to medicine. Their unique chemical compositions and physical properties make them valuable in numerous sectors.
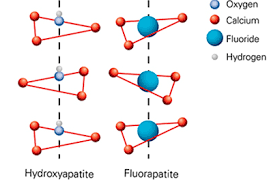
Fluorapatite and hydroxyapatite differ primarily in their chemical constituents; fluorapatite is a phosphate mineral that integrates fluorine, whereas hydroxyapatite is characterized by its hydroxyl content. These differences profoundly impact their physical properties, biological compatibility, and industrial applications. For example, while fluorapatite is extensively used in fertilizers due to its stability, hydroxyapatite is preferred in medical applications for its similarity to human bone.
In terms of their formation and sources, fluorapatite is typically found in igneous rocks and is often mined for its use in commercial products like toothpaste and fertilizers. Hydroxyapatite, on the other hand, is mostly synthesized for high-purity requirements, although it also occurs naturally in bones and teeth, playing a critical role in modern medical treatments and biotechnology.
Basic Properties
Fluorapatite
Chemical Composition
Fluorapatite is a mineral that belongs to the apatite group, primarily consisting of calcium phosphate combined with fluorine. Its chemical formula is Ca₅(PO₄)₃F. This structure incorporates fluorine atoms, which replace the hydroxyl groups found in hydroxyapatite, thus giving fluorapatite its distinctive properties and stability. This substitution enhances the mineral’s resistance to dissolution, making it ideal for various industrial applications.
Physical Characteristics
Fluorapatite is noted for its hardness and durability. On the Mohs scale of mineral hardness, it typically scores around 5, which denotes a medium hardness suitable for various uses, including in abrasives and milling processes. The mineral commonly forms hexagonal crystals and can appear in colors ranging from green to blue, often with a glassy or translucent sheen. These physical attributes not only contribute to its aesthetic value but also affect its utility in scientific and industrial fields.
Hydroxyapatite
Chemical Makeup
Hydroxyapatite, with the chemical formula Ca₅(PO₄)₃(OH), is primarily made up of calcium phosphate, similar to fluorapatite, but with hydroxyl groups in place of fluorine. This composition closely resembles that of human bone, which is approximately 70% hydroxyapatite. This similarity is what makes hydroxyapatite extremely valuable in medical applications, particularly in orthopedics and dentistry.
Physical Properties
Hydroxyapatite is less dense than fluorapatite and has a lower hardness, scoring about 5 on the Mohs scale, which makes it more prone to degradation under stress. However, its biocompatibility — or the ability to coexist with living tissues without causing harm — is a key feature. Hydroxyapatite crystals are also typically smaller and less defined in shape, which aids in its use in medical implants and tissue engineering where fine, granular powders are often required.
Formation and Sources
Fluorapatite Origins
Geological Formation
Fluorapatite forms in a variety of geological environments, primarily through igneous and metamorphic processes. In igneous rocks, it crystallizes from cooling magma, and in metamorphic rocks, it forms from the alteration of phosphate minerals under high temperature and pressure. These processes contribute to the mineral’s wide distribution and abundance in the Earth’s crust.
Common Locations
Significant deposits of fluorapatite are found globally, with notable concentrations in Brazil, Canada, Russia, and parts of Africa. These locations are not only rich in natural resources but also play a crucial role in the global mining industry, which extracts fluorapatite for use in fertilizers, chemicals, and other products.
Hydroxyapatite Origins
Biological Formation
In nature, hydroxyapatite is most commonly found as a component of bones and teeth, where it provides structural rigidity and strength. The mineral forms biologically through cellular processes that deposit calcium phosphate in a structured matrix, which is critical for the growth and maintenance of skeletal tissues.
Synthetic Production
For industrial and medical applications, hydroxyapatite is often synthesized in laboratories to achieve high purity and specific particle sizes. Methods such as wet chemical deposition or hydrothermal synthesis allow for the creation of hydroxyapatite that meets strict standards required for medical implants and other uses.
Applications in Industry
Fluorapatite Uses
Agriculture
In agriculture, fluorapatite is primarily used as a source of phosphate in fertilizers. Its slow release of nutrients helps in the prolonged feeding of crops, which improves yield and sustains soil health. Its role in sustainable agriculture practices is significant due to its efficiency and low environmental impact compared to other synthetic fertilizers.
Ceramics
Fluorapatite is also employed in the ceramics industry. It is used to produce glazes that enhance the appearance and durability of ceramic products. The unique properties of fluorapatite help in controlling the melting behavior of ceramic glazes, leading to improved quality and finish of ceramic goods.
Hydroxyapatite Uses
Medicine
In medicine, hydroxyapatite coatings are used to promote bone growth around prosthetic implants. These coatings improve the integration of implants with existing bone tissue, reducing the risk of implant rejection and enhancing the long-term success of surgical procedures.
Dentistry
Dentists use hydroxyapatite in a variety of applications, including as a filling material and in toothpaste. It helps in the remineralization of teeth, providing a natural form of protection against decay and erosion. This makes hydroxyapatite a critical component in preventive dental care and therapy.
Environmental Impact
Fluorapatite
Mining Effects
The extraction of fluorapatite, primarily through mining operations, has significant environmental implications. These activities often disrupt local ecosystems, leading to habitat destruction and biodiversity loss. Mining processes can also result in substantial soil erosion and contamination of water bodies with chemicals used during the extraction and processing stages. Such impacts necessitate stringent environmental management practices to mitigate damage and preserve ecological balance.
Sustainability Concerns
Concerns over the sustainability of fluorapatite mining are prominent, given its role in producing phosphate fertilizers critical for global agriculture. The depletion of high-quality phosphate reserves raises alarms about the long-term availability and environmental cost of continuing at current extraction rates. Efforts to develop more sustainable mining practices and recycling phosphates from waste sources are crucial to reducing the environmental footprint of fluorapatite usage.
Hydroxyapatite
Biocompatibility
Hydroxyapatite’s biocompatibility is a major advantage, particularly in medical applications. Unlike other synthetic materials, it does not provoke a significant immune response, making it safe for use in implants and prosthetics. This compatibility with human tissue not only minimizes complications but also enhances the healing processes, making it a preferred material in bone surgeries and dental repairs.
Waste Management
The production and use of synthetic hydroxyapatite, especially in high volumes for medical applications, require effective waste management strategies. Manufacturing residues and old prosthetic materials need proper handling to avoid environmental contamination. Recycling used hydroxyapatite, although challenging, is being explored as a way to reduce waste and make the production process more sustainable.
Economic Aspects
Fluorapatite Market
Global Demand
The global demand for fluorapatite is primarily driven by its use in fertilizers and toothpaste production. As the global population grows and agricultural output needs increase, the demand for effective fertilizers rises, boosting the fluorapatite market. The need for sustainable agricultural practices also influences market dynamics, pushing for innovations in fertilizer technologies that rely on fluorapatite.
Price Trends
The price of fluorapatite is influenced by various factors, including mining costs, regulatory policies, and market demand. Prices have fluctuated in recent years due to geopolitical tensions and economic instabilities in key mining regions. Moreover, environmental regulations have also impacted production costs, affecting the overall market pricing of fluorapatite-based products.
Hydroxyapatite Market
Consumption Patterns
Hydroxyapatite consumption is largely dominated by the medical sector, where it is used in implants, reconstructions, and dental products. The growing elderly population worldwide and the increasing prevalence of bone-related diseases have propelled the demand for hydroxyapatite. Additionally, its use in cosmetic dentistry and orthopedics continues to expand, further stimulating market growth.
Growth Projections
The hydroxyapatite market is projected to grow significantly in the coming years. Advances in medical technology, increasing acceptance of hydroxyapatite in various therapeutic applications, and the rising focus on orthopedic and dental health are key drivers of this growth. Emerging markets in Asia and Latin America are particularly promising due to increasing healthcare investments and growing awareness about advanced treatment options.
Research and Innovations
Fluorapatite Advances
New Technologies
Innovative technologies in fluorapatite processing and application are transforming its use, particularly in the agricultural sector. Techniques such as controlled-release fertilizers and nanotechnology-enhanced products are being developed to increase the efficiency and environmental friendliness of fluorapatite-based fertilizers. Such advancements promise to optimize resource use and reduce ecological impacts.
Future Prospects
The future of fluorapatite looks promising with the exploration of its use in new fields such as renewable energy — particularly in the production of photovoltaic cells — and in environmental remediation projects. Research into how fluorapatite can be used to trap heavy metals and radioactive elements offers potential for significant environmental benefits.
Hydroxyapatite Advances
Recent Studies
Recent studies have focused on improving the synthesis and application of hydroxyapatite in medicine. Innovations include better integration techniques with human tissue and the development of composite materials that combine hydroxyapatite with other biocompatible substances to enhance performance and functionality in implants.
Potential Applications
The potential applications for hydroxyapatite are expanding into fields such as drug delivery systems and antibacterial treatments. Research is underway to use hydroxyapatite nanoparticles as carriers for therapeutic agents, offering targeted drug delivery that could revolutionize treatment methodologies for various diseases. Additionally, its antibacterial properties are being harnessed to create more effective and safer medical and dental products.
Frequently Asked Questions
What is Fluorapatite?
Fluorapatite is a mineral form of calcium phosphate where fluorine is incorporated into the crystal lattice. It is commonly used in agricultural fertilizers and in the production of fluoride toothpastes due to its stability and slow release of fluoride ions.
How is Hydroxyapatite Used in Medicine?
Hydroxyapatite is extensively used in the medical field, particularly in orthopedics and dentistry. It serves as a coating for metal implants in bone surgery and as a bone graft material due to its excellent biocompatibility and similarity to human bone tissue.
Are There Environmental Concerns Associated with Fluorapatite?
Yes, the mining and processing of fluorapatite can have environmental impacts, including habitat destruction and pollution from chemical runoff. Sustainable mining practices and proper waste management are crucial to mitigating these effects.
Can Hydroxyapatite Help in Bone Healing?
Absolutely. Hydroxyapatite is pivotal in promoting bone growth and healing. Its composition closely mimics that of natural bone, making it ideal for use in bone grafts, dental implants, and tissue engineering.
Conclusion
Fluorapatite and hydroxyapatite are minerals with significant importance in both natural and synthetic forms, influencing sectors as diverse as agriculture and medicine. Their unique properties cater to specific needs—fluorapatite being pivotal in sustainable agriculture and hydroxyapatite in medical advancements. As research continues, the potential for new applications and improvements in existing technologies appears promising.
The exploration of these minerals not only enhances our scientific understanding but also contributes to advancements in healthcare and environmental management. The continued study and responsible use of fluorapatite and hydroxyapatite will likely provide crucial benefits to society, underlining the importance of these minerals in our daily lives and for future generations.