Iron plays a pivotal role in numerous industrial and biological processes, distinguishing itself through various compounds that exhibit unique properties. Among these, ferric sulphate and ferrous sulphate stand out due to their widespread applications and chemical characteristics. These compounds, derived from different oxidation states of iron, offer a fascinating glimpse into the world of inorganic chemistry and its practical implications in our daily lives.
The difference between ferric and ferrous sulphate primarily lies in their iron ions’ oxidation states; ferric sulphate contains iron in a +3 oxidation state, while ferrous sulphate features iron in a +2 state. This fundamental distinction influences their chemical behavior, uses, and applications across various industries. Such differences underscore the importance of understanding each compound’s unique characteristics to leverage their benefits effectively.
Exploring ferric and ferrous sulphate reveals their significance in processes ranging from water purification to the synthesis of other chemicals. Ferric sulphate, with its higher oxidation state, serves as a potent coagulant in water treatment, whereas ferrous sulphate, often seen in supplement form, plays a crucial role in addressing iron deficiency. The contrasting properties of these compounds illustrate the versatility of iron as an element, making them invaluable in both environmental management and healthcare.
Iron Sulphate Basics
Iron sulphate represents a fascinating intersection of chemistry and utility, providing a foundation for numerous applications in our world. It comes in different forms, each with unique properties and uses, derived from the two primary states of iron: ferric and ferrous.
Types of Iron
Elemental Iron Overview
Iron, a metallic element, is found abundantly in the Earth’s crust. Known for its magnetic properties and metallic luster, elemental iron is a key component in the manufacturing of steel, one of the most important materials in construction and manufacturing globally. In its pure form, iron is relatively soft but becomes significantly harder and stronger when alloyed with carbon.
Ferric vs. Ferrous Ions
The distinction between ferric (Fe^3+) and ferrous (Fe^2+) ions lies in their oxidation states. Ferric ions have lost three electrons, while ferrous ions have lost two. This difference is crucial as it affects the ions’ chemical reactivity and the types of compounds they can form. Ferric ions are typically seen in oxidizing environments, while ferrous ions are more common in reducing conditions.
Sulphate Introduction
Sulphate, a compound containing the sulphate ion (SO4^2-), plays a significant role in various chemical reactions and processes. It’s known for its ability to form salts with a wide range of metallic elements, including iron.
Role of Sulphate in Compounds
In chemical compounds, sulphate acts as a stabilizing anion, allowing for the formation of diverse salts. These sulphate-based salts are crucial in numerous industrial and biological processes due to their solubility in water and chemical stability.
Common Sulphate Compounds
Examples of common sulphate compounds include gypsum (CaSO4·2H2O), used in building materials, and magnesium sulphate (MgSO4), known as Epsom salts, used in bath salts and as a magnesium supplement.
Ferric Sulphate
Ferric sulphate, with the chemical formula Fe2(SO4)3, is an inorganic compound that has significant applications, particularly in water treatment.
Chemical Structure
Composition and Formula
Ferric sulphate consists of iron in the +3 oxidation state and the sulphate ion. Its molecular formula, Fe2(SO4)3, denotes two iron (Fe) atoms and three sulphate (SO4) groups.
Physical Properties
This compound appears as a brown-yellow crystalline solid at room temperature and is highly soluble in water. Its solubility is a key feature that enables its use in various applications, especially in water treatment processes.
Production Methods
Industrial Preparation
- Roasting: Iron ores are roasted with sulphuric acid, producing ferric sulphate and other by-products.
- Acidification: Solutions of ferrous sulphate are oxidized and acidified to form ferric sulphate.
Laboratory Synthesis
- Oxidation: Ferrous sulphate is oxidized using hydrogen peroxide in the presence of an acid to yield ferric sulphate.
- This method is beneficial for producing small quantities of high-purity ferric sulphate for research and testing.
Uses and Applications
Water Treatment Processes
Ferric sulphate is extensively used as a coagulant in water treatment facilities. It helps to remove impurities, such as suspended particles and bacteria, from water, making it safe for consumption and use.
- Coagulation: When added to water, it causes contaminants to clump together, making them easier to remove.
- Flocculation: This process enhances the coagulation process, resulting in clearer water.
Other Industrial Applications
- Dyeing Fabrics: Used as a mordant in the dyeing process to ensure dyes bond well with fabrics.
- Manufacturing: Involved in the production of other chemicals, such as pigments and other types of iron salts.

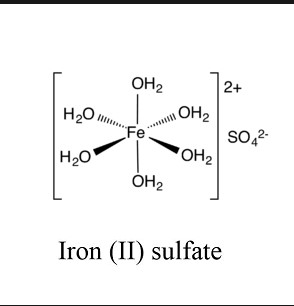
Ferrous Sulphate
Ferrous sulphate, an essential compound with significant applications, plays a pivotal role in agriculture, medicine, and various industries. Understanding its chemical composition, production techniques, and applications reveals its importance.
Chemical Composition
Formula and Structure
Ferrous sulphate, with the chemical formula FeSO4, consists of one iron (Fe) atom in the +2 oxidation state and one sulphate (SO4) group. This compound is known for its greenish or white crystals when hydrated, showcasing its distinct physical characteristics.
Physical Characteristics
- Appearance: It commonly appears as a blue-green crystalline solid in its heptahydrate form (FeSO4·7H2O).
- Solubility: Ferrous sulphate is highly soluble in water, a property that significantly influences its applications and effectiveness in various sectors.
Production Techniques
Extraction and Manufacturing
The production of ferrous sulphate involves several methods, including:
- Direct extraction: From iron-rich ores using sulfuric acid, which yields ferrous sulphate directly.
- By-products: Often obtained as a by-product in the steel manufacturing process, providing an efficient recycling pathway.
Lab-Scale Production
For smaller quantities with high purity, ferrous sulphate can be produced through chemical reactions, including:
- Dissolving iron: Iron filings are dissolved in dilute sulfuric acid, followed by crystallization to obtain the pure compound.
Applications
Agriculture and Horticulture
Ferrous sulphate is widely used in agriculture to:
- Correct iron deficiency in plants, preventing chlorosis and promoting healthy growth.
- Soil amendment: It reduces soil pH, making it more suitable for acid-loving plants.
Medical Uses
In medicine, ferrous sulphate is primarily used as a dietary supplement to treat:
- Iron deficiency anemia: It provides a readily absorbable source of iron, essential for hemoglobin production.
Key Differences
Chemical Properties
Oxidation States and Reactivity
- Ferrous ions (Fe^2+) are more reducible than ferric (Fe^3+), affecting their reactivity and chemical interactions.
- Solubility and Stability: Ferrous sulphate is generally more soluble but less stable than ferric sulphate, tending to oxidize to ferric form upon exposure to oxygen.
Health and Safety
Handling Precautions
- Protective gear is necessary due to the risk of skin irritation and potential toxicity if ingested.
- Storage: Ferrous sulphate should be stored in a cool, dry place to prevent oxidation.
Environmental Impacts
- Proper disposal is crucial to avoid water and soil contamination. Despite its use in treating water, excessive amounts can harm aquatic life.
Economic Aspects
Cost Comparison
- Ferrous sulphate is often less expensive than ferric sulphate due to its availability and the ease of production.
- Availability and Sourcing: It is readily available, partly because it can be derived as a by-product of other industrial processes.
Comparative Analysis
Industrial Use
Sector-Specific Applications
- Water treatment: Ferrous sulphate is used for phosphate removal.
- Colorant in Cement: Provides a greener tint to concretes and mortars.
Efficiency and Effectiveness
- Agriculture: Highly effective in treating iron chlorosis, improving crop yield and quality.
- Medicine: Essential in preventing and treating iron deficiency anemia, showcasing its effectiveness in healthcare.
Environmental Impact
Eco-Friendliness
- When used responsibly, ferrous sulphate can improve soil quality and plant health with minimal environmental impact.
- Disposal Considerations: Careful disposal methods are necessary to prevent environmental contamination, highlighting the need for sustainable practices.
Frequently Asked Questions
What is Ferric Sulphate Used For?
Ferric sulphate is primarily used as a coagulant in wastewater treatment plants to remove impurities. It facilitates the aggregation of contaminants, making them easier to filter out. Additionally, it’s employed in the production of other chemicals and as a coloring agent in some applications, showcasing its versatility in industrial processes.
How is Ferrous Sulphate Produced?
Ferrous sulphate is produced through several methods, including the treatment of iron with sulfuric acid under specific conditions. It can also be obtained as a by-product of the titanium dioxide manufacturing process. This compound is further refined and processed to meet various industrial and pharmaceutical standards.
Can Ferrous Sulphate be Used in Agriculture?
Yes, ferrous sulphate is widely used in agriculture to correct iron deficiency in plants, a condition known as chlorosis, which causes yellowing of the leaves due to insufficient chlorophyll. It’s also used to lower soil pH, making it more acidic, which can benefit certain types of plants by improving nutrient absorption.
What are the Safety Considerations for Handling Ferric and Ferrous Sulphate?
Both ferric and ferrous sulphate should be handled with care, as they can be harmful if ingested or if they come into contact with skin and eyes. Protective clothing, gloves, and eye protection are recommended when handling these chemicals. Proper storage and disposal methods should be followed to minimize environmental impact and ensure safety.
Conclusion
Ferric and ferrous sulphate serve as quintessential examples of how variations in chemical composition can lead to a wide array of applications and functionalities. The insight into their differences not only enriches our understanding of chemical processes but also highlights the importance of choosing the right compound for specific industrial, environmental, or health-related purposes. As we continue to explore the nuances of these substances, their distinct roles in our world become increasingly clear, underlining the beauty and complexity of chemistry in practical applications.
Their application extends beyond mere industrial use, touching aspects of environmental conservation and health, which emphasizes the critical role these compounds play in modern society. Recognizing and appreciating the differences between ferric and ferrous sulphate allows for more informed decisions, better industrial practices, and a deeper appreciation of the intricate balance between chemistry and its application in the real world.

