Chlorinated hydrocarbons, including ethyl chloride and allyl chloride, represent a crucial class of chemicals extensively used in various industrial and medical applications. Each compound, characterized by unique properties and structures, plays a vital role in the synthesis of other chemical products. Despite their similar origins, these substances exhibit distinct behaviors and uses, which are significant for different applications.
Ethyl chloride and allyl chloride differ primarily in their chemical structure and resultant physical properties. Ethyl chloride, a simple chlorinated hydrocarbon, is known for its role as a local anesthetic in minor medical procedures, while allyl chloride serves as a precursor in the manufacture of other significant compounds, including pharmaceuticals and pesticides. The differentiation in their applications stems from their molecular composition and the presence of the double bond in allyl chloride, which is absent in ethyl chloride.
The study of these chemicals extends beyond their applications, touching upon aspects such as production methods, health and safety issues, environmental impact, and regulatory considerations. Understanding these elements is crucial for their effective and safe use in various sectors, ensuring they contribute positively to industries and healthcare without significant adverse environmental impacts.
Chemical Profiles
Ethyl Chloride
Basic Structure
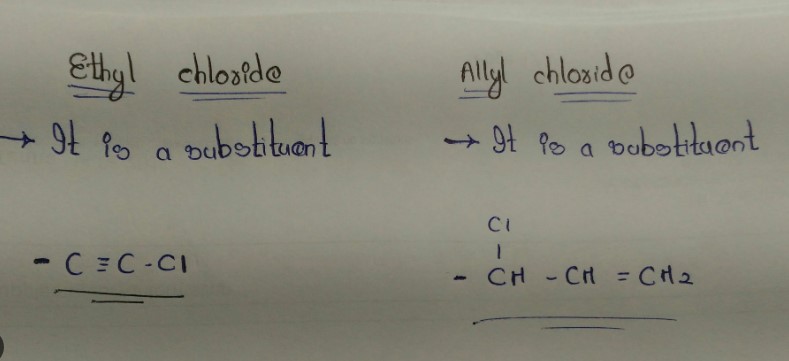
Ethyl chloride, scientifically known as chloroethane, features a simple molecular structure consisting of two carbon atoms and a chlorine atom attached to one of these carbons. The molecular formula for ethyl chloride is C2H5Cl. This configuration results in a highly volatile molecule, primarily used in quick applications due to its rapid evaporation from the liquid form.
Physical Properties
Ethyl chloride is a colorless, flammable gas at room temperature with a faintly sweet odor. It has a boiling point of approximately -12.3 degrees Celsius, which allows it to be used in liquid form under pressure or as a refrigerated liquid. It is slightly soluble in water, but mixes well with most organic solvents.
Common Uses
- Medical Applications: Ethyl chloride is predominantly used as a local anesthetic in minor surgical procedures. It quickly numbs the targeted area when sprayed.
- Industrial Applications: In the industrial sphere, ethyl chloride serves as a refrigerant, a solvent for fats, oils, and waxes, and as a chemical intermediate in the production of tetraethyl lead.
Allyl Chloride
Basic Structure
Allyl chloride is a compound with the formula C3H5Cl. It differs from ethyl chloride by having a double bond between the second and third carbon atoms, which makes it more reactive. This reactivity is crucial for its role in various chemical synthesis processes.
Physical Properties
As a colorless liquid with a pungent odor, allyl chloride has a boiling point of about 44-46 degrees Celsius. It is less soluble in water compared to ethyl chloride but reacts more readily with other compounds due to its double bond.
Common Uses
- Chemical Synthesis: The primary use of allyl chloride is as an intermediate in the production of other chemicals, notably epichlorohydrin, which is used to make epoxy resins.
- Specialty Applications: Due to its reactive nature, it is also used in the synthesis of pharmaceuticals and other specialty chemicals.

Production Methods
Synthesis of Ethyl Chloride
Industrial Processes
- Direct Hydration of Ethylene: Ethylene gas reacts with hydrochloric acid to produce ethyl chloride. This method is commonly employed in large-scale production due to its efficiency.
- Ethanol Chlorination: Ethanol can be reacted with hydrochloric acid or hydrogen chloride under specific conditions to yield ethyl chloride.
Laboratory Methods
- Small Scale Synthesis: In laboratories, ethyl chloride is often synthesized by reacting ethanol with sodium chloride and sulfuric acid, a method suitable for educational purposes and small-scale applications.
Synthesis of Allyl Chloride
Industrial Processes
- From Propylene: The most common industrial method involves the chlorination of propylene at high temperatures. This reaction must be carefully controlled to prevent the formation of undesirable byproducts.
Laboratory Methods
- Detailed Synthesis: Laboratory synthesis of allyl chloride might involve more precise control of reaction conditions to study reaction mechanisms or for teaching purposes.
Applications
Uses of Ethyl Chloride
Medical Applications
- Local Anesthetic: Widely used for its rapid numbing effect in minor medical procedures such as sports injuries and small surgical interventions.
Industrial Applications
- Manufacture of Other Chemicals: Ethyl chloride is used as a precursor in the synthesis of tetraethyl lead, once a widely used antiknock agent in gasoline.
Uses of Allyl Chloride
Chemical Synthesis
- Precursor to Epichlorohydrin: Allyl chloride is vital in manufacturing epichlorohydrin, a key ingredient in producing epoxy resins, which are essential in the aerospace, automotive, and construction industries.
Specialty Applications
- Pharmaceutical Manufacturing: It is also used in the synthesis of several pharmaceuticals, offering structural components essential for active drug ingredients.
Health and Safety
Risks with Ethyl Chloride
Exposure Risks
- Inhalation: Can cause respiratory discomfort and, in high concentrations, central nervous system effects.
- Skin Contact: Can cause frostbite due to its extremely low boiling point when evaporating.
Handling and Storage
- Storage Requirements: Ethyl chloride should be stored in a cool, well-ventilated area away from direct sunlight and ignition sources.
- Safety Equipment: Proper protective clothing and eye protection should always be used when handling ethyl chloride.
Risks with Allyl Chloride
Exposure Risks
- Inhalation: Exposure can irritate the respiratory system and prolonged contact might lead to more severe health issues.
- Skin Contact: Can cause irritation and, in severe cases, chemical burns.
Handling and Storage
- Safety Protocols: Due to its reactivity and flammability, allyl chloride must be handled with extreme care.
- Storage Recommendations: It should be stored in airtight containers away from incompatible substances and heat sources to prevent accidental reactions.

Environmental Impact
Ethyl Chloride and Ecology
Breakdown in Nature
Ethyl chloride is known for its high volatility, which means it tends to evaporate quickly when exposed to air, limiting its persistence in soil and water environments. However, in its gaseous state, it can contribute to atmospheric pollution. In terms of breakdown, ethyl chloride generally degrades through reactions with atmospheric hydroxyl radicals, a process that eventually converts it to simpler molecules like hydrogen chloride and carbon dioxide.
Environmental Risks
The primary concern with ethyl chloride in the environment revolves around its volatility and flammability. Accidental releases can lead to the formation of explosive mixtures in the air, posing risks not only to environmental health but also to human safety. Although it does not accumulate in the biosphere, its presence in the atmosphere can contribute to ozone layer depletion, warranting careful handling and regulatory compliance to prevent emissions.
Allyl Chloride and Ecology
Breakdown in Nature
Allyl chloride also displays a high rate of evaporation but is more reactive than ethyl chloride. It breaks down in the environment primarily through reaction with water and soil minerals, leading to the formation of hydrochloric acid and other less stable intermediates that can further degrade into harmless substances. The rate and nature of these reactions can vary significantly depending on local environmental conditions such as pH and temperature.
Environmental Risks
The reactivity of allyl chloride poses distinct environmental risks, primarily through its potential to contaminate groundwater and soil during spills. Its breakdown products, which can be acidic, might affect the pH of local ecosystems, adversely impacting plant and aquatic life. Furthermore, the toxic nature of the compound requires strict regulatory oversight to ensure that any accidental releases are quickly managed and remediated.
Regulatory Landscape
Ethyl Chloride Regulations
National Guidelines
In the United States, ethyl chloride is regulated under the Clean Air Act, which mandates the reporting of emissions that could potentially contribute to local air quality degradation. Manufacturers and users of ethyl chloride must adhere to strict guidelines on handling, storage, and disposal to minimize environmental release. The Occupational Safety and Health Administration (OSHA) also sets exposure limits to protect workers from the potential health effects of inhalation.
International Standards
Globally, ethyl chloride is subject to various regulations that oversee its transport, use, and disposal. The Montreal Protocol, which aims at phasing out substances that deplete the ozone layer, monitors the use of ethyl chloride due to its volatile nature and potential to contribute to atmospheric changes. Compliance with international standards is crucial for countries that export or import ethyl chloride, ensuring that these practices align with global environmental goals.
Allyl Chloride Regulations
National Guidelines
In the U.S., allyl chloride is listed as a High Priority Substance under the Toxic Substances Control Act (TSCA), which requires industries to report detailed information about how much they produce and discharge into the environment. This regulation helps in monitoring and controlling the potential risks associated with its use. Safety protocols for handling allyl chloride are stringent, emphasizing protective equipment and emergency response plans to prevent occupational exposure.
International Standards
Internationally, regulations regarding allyl chloride focus on reducing risks associated with its toxicity and reactivity. The chemical is often scrutinized under global chemical safety agreements like the Rotterdam Convention, which facilitates information exchange on hazardous chemicals and promotes safe handling practices. Compliance with these standards ensures that allyl chloride is used and transported in ways that minimize environmental and health risks.
Frequently Asked Questions
What is Ethyl Chloride?
Ethyl chloride is a volatile, flammable organic compound with a low boiling point, primarily used in medicine for its anesthetic properties. It acts quickly to numb the application area, making it ideal for short medical procedures.
What is Allyl Chloride?
Allyl chloride is a more reactive chlorinated hydrocarbon compared to ethyl chloride, primarily used as a chemical intermediate. It is crucial in the synthesis of pharmaceuticals, pesticides, and other organic compounds.
How are Ethyl and Allyl Chloride Produced?
Ethyl chloride is typically produced through the reaction of ethanol with hydrochloric acid or by ethane chlorination. Allyl chloride, on the other hand, is primarily manufactured by the reaction of propylene with chlorine during high-temperature chlorination processes.
What are the Main Uses of Allyl Chloride?
The main use of allyl chloride is as an intermediate in the production of other chemicals. It is particularly valuable in synthesizing epichlorohydrin, which is used to make glycerol, plastics, and epoxy resins.
How Do Ethyl and Allyl Chloride Impact the Environment?
Both chemicals pose risks to the environment if not managed properly. Ethyl chloride can contribute to air pollution due to its volatile nature, while allyl chloride can lead to soil and water contamination due to its reactivity and toxicity.
Conclusion
The distinct properties and applications of ethyl chloride and allyl chloride highlight the importance of tailored usage and management strategies for each. As chemicals that influence a broad spectrum of industries, from healthcare to manufacturing, understanding their differences is essential for optimizing their benefits while minimizing potential risks.
Future considerations for the use and regulation of these compounds will likely focus on enhancing safety measures and developing more sustainable production methods. As we advance, the role of scientific innovation and regulatory frameworks will be crucial in maintaining the balance between utilizing these essential chemicals and protecting public and environmental health.

