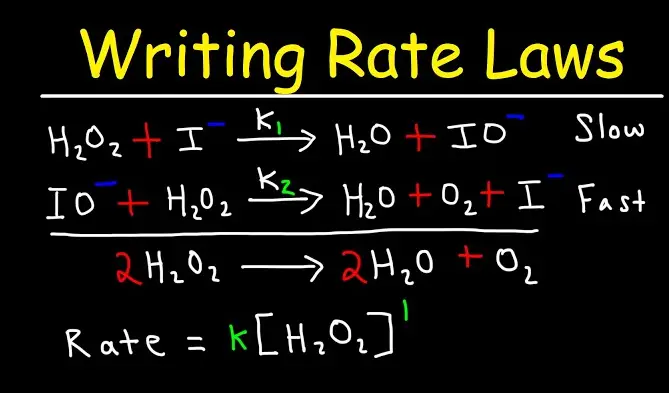
Chemical reactions are complex processes where molecules interact to form new products, often proceeding through several steps. Each of these steps has unique features and impacts on the overall reaction rate. In the study of chemical kinetics, two critical concepts are the elementary step and the rate determining step, each playing a distinct role in how chemical reactions unfold.
The elementary step in a reaction mechanism is a single reaction stage where reactants convert directly to products, typically without intermediary stages. The rate determining step, conversely, is the slowest step in a sequence of reactions that dictates the overall reaction speed. It is the bottleneck of the process, controlling how quickly the reaction progresses toward completion.
Grasping these concepts is crucial for chemists and industries that rely on precise reaction mechanisms to innovate or optimize products. These steps not only determine the efficiency of chemical processes but also influence the design and improvement of catalytic systems, pharmaceuticals, and materials science innovations.

Elementary Steps Explained
Definition and Characteristics
An elementary step in chemical kinetics is a fundamental process where reactants undergo a single, indivisible transformation to become products. These steps are the building blocks of more complex reaction mechanisms. Each elementary step is characterized by the following traits:
- Directness: The conversion of reactants to products occurs in a single, unbroken sequence.
- Stoichiometry: The molecular change in an elementary step matches the stoichiometric equation of the reaction, meaning there are no intermediate compounds not accounted for in the overall reaction equation.
- Concreteness: These steps do not imply any hidden or intermediate stages within their progression.
Types of Elementary Reactions
Elementary reactions can be categorized based on the number of molecules participating in the reaction:
- Unimolecular: Involving the transformation of a single molecule, such as isomerization.
- Bimolecular: Two reactant molecules collide and react to form the products, common in most chemical reactions.
- Termolecular: Involves three reacting molecules, which are less common due to the low probability of three molecules colliding at the same time.
Role in Reaction Mechanisms
Elementary steps play a critical role in the overall understanding of chemical mechanisms. By analyzing these steps, chemists can:
- Predict Products: Determine what products are likely to form from given reactants.
- Understand Rates: Gain insights into the speed of the reaction based on the complexities of each elementary step.
- Optimize Conditions: Adjust temperature, pressure, or catalysts to achieve the most efficient reaction path.
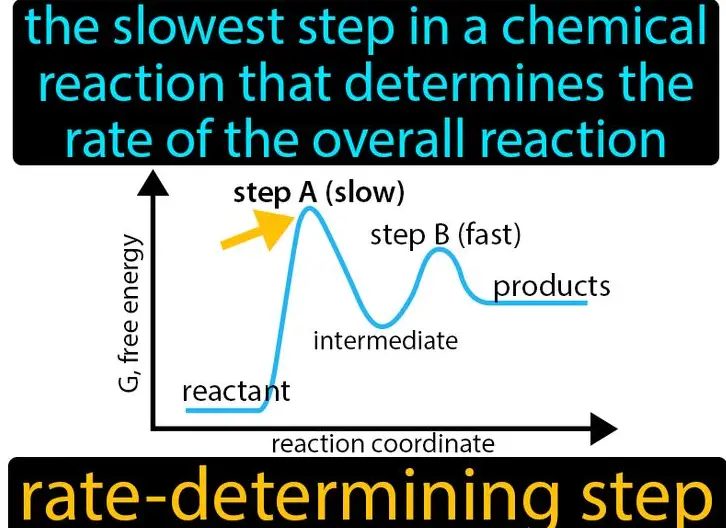
Rate Determining Step
Definition and Importance
The rate determining step is the slowest step within a reaction mechanism, acting as the bottleneck that controls the overall rate of the reaction. This step is crucial because:
- It dictates the kinetics of the reaction.
- It determines the energy required to progress the reaction.
- It influences the design of chemical processes by pinpointing where enhancements are most needed.
How It Influences Reaction Rates
The rate determining step significantly impacts the reaction rate by:
- Setting Speed Limits: The overall reaction cannot proceed faster than this slowest step.
- Energy Consumption: This step typically has the highest activation energy barrier to overcome.
- Reaction Dynamics: Modifies how reactants are consumed and products are formed over time.
Identifying the Rate Determining Step in a Mechanism
To identify the rate determining step, chemists often:
- Analyze rate laws and reaction orders derived from experimental data.
- Monitor concentration changes over time to see which step lags behind.
- Use theoretical calculations and simulations to predict energy barriers.
Comparing Steps
Key Differences
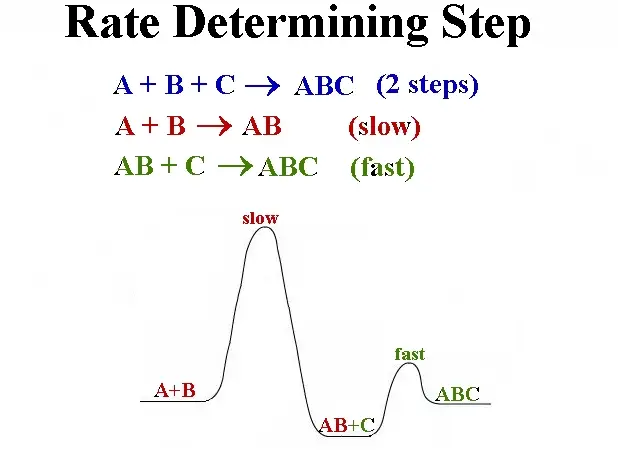
While both types of steps are integral to reaction mechanisms, their roles and characteristics differ significantly:
- Speed: Elementary steps can be fast or moderate, while the rate determining step is always the slowest.
- Function: Elementary steps describe specific changes; the rate determining step governs the reaction pace.
- Multiplicity: There can be many elementary steps, but typically only one rate determining step under constant conditions.
Examples in Common Chemical Reactions
In the synthesis of ammonia (Haber process), multiple elementary steps occur, but the adsorption of nitrogen is the rate determining step due to its high energy requirement. Similarly, in the decomposition of hydrogen peroxide, various steps happen rapidly, but the formation of the hydroxyl radical often controls the rate.
Diagrammatic Representations
Diagrams and flowcharts are effective in illustrating these steps. They show:
- Pathways: Visualize how each elementary step leads to the next.
- Energy Profiles: Graphical representations of energy changes through the reaction sequence, highlighting the rate determining step with the highest peak.
Factors Influencing Rate Steps
Energy Barriers and Reaction Pathways
Energy barriers are crucial determinants of the rate at which chemical reactions proceed. These barriers represent the minimum energy that reactants need to initiate a reaction. The height of the energy barrier directly influences the reaction rate; higher barriers result in slower reactions. Reaction pathways, the routes taken by molecules as they transform from reactants to products, can vary in complexity, with each path having its own characteristic energy profile.
- Single-step reactions often have a straightforward pathway with a clear energy barrier.
- Multi-step reactions involve several transitions, each with its own energy requirement, but the highest barrier defines the rate determining step.
Catalysts and Their Effects
Catalysts play a transformative role in chemical reactions by lowering energy barriers, thus accelerating reaction rates. They achieve this without being consumed in the reaction, making them efficient tools for industrial and laboratory processes. Catalysts work by:
- Providing an alternative pathway with a lower activation energy.
- Stabilizing the transition state of the reaction.
- Increasing the number of effective collisions between reactants.
Temperature and Concentration Dependencies
The rate of a chemical reaction is also influenced by temperature and concentration of reactants:
- Temperature: Increasing the temperature generally increases reaction rates. Higher temperatures provide more energy to the reactants, helping them overcome energy barriers more readily.
- Concentration: Higher concentrations of reactants lead to more collisions and, consequently, an increased chance of overcoming the energy barrier.
Impact on Chemical Kinetics
Mathematical Modeling of Rate Determining Steps
Mathematical models are essential tools in chemical kinetics, helping to predict and analyze the behavior of reactions based on the rate determining step. These models use differential equations to describe how concentrations of reactants and products change over time. Key considerations include:
- Rate laws that incorporate the concentration and order of reactants.
- Time-dependent studies that simulate reaction progress under varying conditions.
Predicting Reaction Outcomes
Understanding the rate determining step allows chemists to accurately predict how a reaction will proceed, which is crucial for designing experiments and processes. Predictions based on kinetic models can inform:
- The yield of a reaction.
- The time required to reach completion.
- The conditions necessary for optimal product formation.
Case Studies from Industrial Processes
Several industrial processes have been optimized by applying principles of rate determining steps and kinetics. For example:
- Ammonia Synthesis: The Haber process was enhanced by identifying and optimizing the rate determining step involving nitrogen fixation.
- Petroleum Refining: Cracking processes depend on understanding the kinetics of carbon-carbon bond breaking, which is often the rate determining step.
Practical Applications
Industrial Synthesis
Chemical kinetics, particularly the knowledge of rate determining steps, is vital for the synthesis of chemicals on an industrial scale. Industries leverage this knowledge to:
- Enhance the efficiency of reactions.
- Reduce energy consumption and costs.
- Scale up laboratory processes to industrial production without loss in yield or quality.
Pharmaceutical Developments
In pharmaceuticals, the synthesis of active ingredients must be precisely controlled to ensure efficacy and safety. Understanding the kinetics of reactions helps in:
- Optimizing reaction conditions to increase purity.
- Minimizing the formation of harmful byproducts.
- Scaling production from milligrams in the lab to kilograms in manufacturing.
Environmental Impact Assessments
The environmental impact of chemical processes can be mitigated by optimizing the rate determining steps to minimize waste and energy usage. Environmental applications include:
- Designing greener catalytic processes that reduce the need for hazardous chemicals.
- Developing methods to breakdown pollutants more effectively in wastewater treatment.
Frequently Asked Questions
What is an elementary step?
An elementary step in a chemical reaction is a single, indivisible process that directly converts reactants into products. These steps occur exactly as depicted by their molecular equation, without any intermediate or hidden stages.
How does a rate determining step affect a reaction?
The rate determining step is the slowest step in a chemical reaction mechanism and thus sets the pace for the entire reaction. It effectively controls the rate at which the reaction proceeds, influencing both the time scale and the efficiency of the process.
Can a reaction have multiple rate determining steps?
Typically, a chemical reaction will have one rate determining step under a given set of conditions. However, changes in temperature, pressure, or reactant concentrations can alter the reaction pathway, potentially shifting the rate determining step to a different stage within the mechanism.
Why is understanding these steps important?
Understanding elementary and rate determining steps is essential for designing efficient chemical processes, enhancing reaction rates, and minimizing unwanted byproducts. This knowledge is particularly vital in industries such as pharmaceuticals, where precise control over reaction conditions can impact drug purity and yield.
Conclusion
The distinction between an elementary step and a rate determining step is foundational in the field of chemical kinetics. These concepts not only aid in the understanding of how reactions proceed but also in the strategic planning and optimization of industrial and laboratory processes. By focusing on the rate determining step, chemists can devise more effective methods to accelerate reactions and improve outcomes, contributing significantly to advancements in technology and medicine.
Exploring these mechanisms further can lead to significant breakthroughs in how we manipulate chemical reactions for various applications, highlighting the importance of continued research and education in chemical kinetics.