Are you struggling to understand the difference between electropositive and electronegative elements? In this blog post, we will explore the definitions of each term and discuss the key differences between them.
By the end of this post, you will have a better understanding of the two concepts and be able to distinguish between them.
Comparing and contrasting electropositive and electronegative elements

Comparing and contrasting electropositive and electronegative elements can be tricky. At the most basic level, electropositive elements are those that have a tendency to give up electrons, while electronegative elements have a tendency to attract electrons. This difference in behavior is due to the different ways in which electrons are distributed within the atoms of these elements.
Electropositive elements, such as the alkali metals, have relatively few electrons in their outer shells, making them more likely to give up electrons and become positively charged. Electronegative elements, such as the halogens, have more electrons in their outer shells, making them more likely to attract electrons and become negatively charged.
This difference in electronegativity can lead to a wide range of reactions, from simple ionic bonds to complex covalent bonds. Understanding the differences between electropositive and electronegative elements can help chemists better predict the reactivity of different elements and design more effective experiments.
Examples of electropositive and electronegative elements
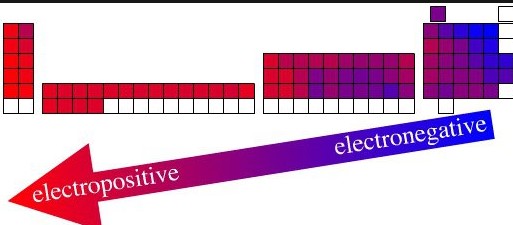
The difference between electropositive and electronegative elements is one of the most important concepts in chemistry. Electropositive elements are those that readily give up electrons to other elements or molecules.
Electronegative elements, on the other hand, are those that attract electrons from other molecules or atoms. Examples of electronegative elements include chlorine, fluorine, and oxygen, all of which readily acquire electrons during chemical reactions.
Understanding the difference between electropositive and electronegative elements is critical to understanding chemical reactions, as it helps explain why certain elements react with one another and form bonds.
Electropositive and electronegative characteristics
The difference between electropositive and electronegative elements is that electropositive elements tend to easily give up electrons, while electronegative elements tend to attract electrons more readily. Electropositive elements, such as alkali metals, are usually found in the left side of the periodic table, while electronegative elements, such as halogens, are typically found on the right side.
This difference in affinity is due to the differences in electronegativity of the elements. The higher the electronegativity of the element, the more likely it is to gain or donate electrons.
The chemical reactions of electropositive and electronegative elements

The difference between electropositive and electronegative elements lies in the nature of their chemical reactions. Electropositive elements are those that tend to give up electrons in chemical reactions, forming positively charged ions.
On the other hand, electronegative elements tend to take up electrons, forming negatively charged ions. As a result, when an electropositive element and an electronegative element interact, the electropositive element will give up its electrons to the electronegative element, creating an ionic bond between them. This bond is what creates the unique properties of many molecules and compounds.
Understanding the difference between electropositive and electronegative elements is essential for understanding the behavior of chemical reactions.
The benefits of understanding electropositive and electronegative elements
Understanding the differences between electropositive and electronegative elements can have a major impact on how we approach chemistry and other related topics. Electropositive elements are those that easily give off electrons, while electronegative elements are those that attract electrons.
This distinction is important when discussing chemical reactions, as the interaction between electrons and atoms will determine the outcome of the reaction. By understanding the differences between electropositive and electronegative elements, we can better predict the outcome of our experiments and make more informed decisions. Additionally, understanding this concept can help us better understand the structure of molecules and the behavior of certain compounds.
Knowing which elements are electropositive or electronegative can help us design more efficient reactions and develop more effective products. Ultimately, understanding the difference between electropositive and electronegative elements can help us further our knowledge of chemistry and the world around us.
Final Touch
In conclusion, the difference between electropositive and electronegative elements is the ability to attract electrons. Electropositive elements have a high affinity for electrons and are generally more reactive, while electronegative elements have a weak affinity for electrons and are less reactive. The difference is important to understand when considering chemical reactions and the transfer of electrons.
The difference is important to understand when considering chemical reactions and the transfer of electrons.

