Analytical chemistry plays a pivotal role in numerous scientific disciplines, determining the composition of materials and substances through various methods. Two fundamental techniques employed for the quantitative determination of nitrogen in organic compounds are the Dumas and Kjeldahl methods. Both methods have long histories and are critical in environmental, pharmaceutical, and food industries for their distinct approaches to nitrogen quantification.
The Dumas method involves combusting the sample in an oxygen-rich environment, converting all nitrogen present into nitrogen gas, which is then quantified. On the other hand, the Kjeldahl method acid-digests the sample, converting nitrogen into ammonium sulfate before it is further processed and quantified. These procedures, though differing in technique and application, provide essential data for analyzing protein content in various substances.
While the Dumas method is admired for its rapid processing time and minimal chemical waste, the Kjeldahl method is celebrated for its exceptional accuracy and reliability in a wider range of samples. Both methods have undergone significant technological advancements, enhancing their efficiency and environmental compatibility, making them indispensable tools in modern analytical chemistry.
Dumas Method
Historical Context
The Dumas method, named after its inventor Jean-Baptiste Dumas, has been a cornerstone in analytical chemistry since the 19th century. Initially developed in 1831, this method revolutionized the way chemists quantified nitrogen in organic substances, setting the stage for modern protein analysis in various industries.
Basic Principles
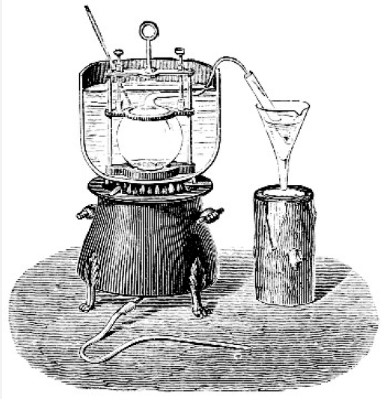
The basic principle behind the Dumas method is the combustion of a sample in an oxygen-rich environment. This process converts all the nitrogen in the sample to nitrogen gas (N2), which is then measured to determine the nitrogen content. This method is noted for its simplicity and speed in handling a wide range of samples.
Equipment and Chemicals
To perform the Dumas method, several key pieces of equipment are required:
- Combustion analyzer: This is the primary instrument used to burn the sample and measure the nitrogen gas.
- Oxygen tank: Supplies the oxygen needed for combustion.
- Ballast: Helps regulate the flow of gases in the analyzer.
The chemicals involved are minimal, primarily oxygen, which is used to create the combustion environment.
Procedure Overview
The process of conducting a Dumas test involves several clear steps:
- Preparation: Weigh a small, dry sample and place it in the combustion analyzer.
- Combustion: Ignite the sample with oxygen to ensure complete combustion.
- Gas analysis: Measure the volume of nitrogen gas produced, which correlates to the nitrogen content.
This method is preferred for its efficiency and reduced chemical waste.
Applications
The Dumas method is widely used in:
- Food industry: For protein content analysis in food products.
- Agricultural science: To study nitrogen levels in soil and plant tissues.
- Environmental monitoring: Assessing nitrogen pollution levels in water.
Kjeldahl Method
Historical Background
Developed by Johan Kjeldahl in 1883, this method quickly became a gold standard for measuring nitrogen, particularly in organic substances. It has been indispensable in environmental science, agriculture, and the food industry, providing detailed insights into protein and nitrogen-containing compounds.
Fundamental Concepts
The Kjeldahl method focuses on the acid digestion of organic material, converting nitrogen into ammonium sulfate. This is then alkalized, and the released ammonia is distilled and quantified. The reliability and accuracy of this method have stood the test of time.
Required Materials
To conduct a Kjeldahl analysis, the following materials are needed:
- Digestion flasks: For containing the sample and acids.
- Distillation unit: To separate ammonia from the digestion mixture.
- Boric acid: To capture the distilled ammonia.
- Indicator solution: Helps in the titration process.
Step-by-Step Process
The steps involved in the Kjeldahl method are:
- Digestion: Add sulfuric acid to the sample in a digestion flask and heat until clear.
- Alkalization: Add sodium hydroxide to make the solution alkaline.
- Distillation: Distill off the ammonia into a boric acid solution.
- Titration: Determine the ammonia content by titration.
Usage in Various Industries
The Kjeldahl method finds applications in:
- Food processing: For nutritional labeling and quality control.
- Pharmaceuticals: To ensure the correct formulation of drugs.
- Research: In biochemistry for studying nitrogen-fixing in plants.
Comparison of Methods
Accuracy and Precision
While both methods are precise, the Kjeldahl method is often considered more accurate for complex samples, where other elements might interfere with the nitrogen measurement in the Dumas method.
Speed and Efficiency
The Dumas method is generally faster, taking minutes per sample compared to hours with the Kjeldahl method. This makes it suitable for high-throughput environments.
Cost Implications
Kjeldahl equipment tends to be more expensive and requires more reagents, which can increase operational costs. In contrast, the Dumas method has lower ongoing costs due to fewer consumables.
Environmental Considerations
The Dumas method scores higher in environmental friendliness, as it involves fewer chemicals and generates less waste. Meanwhile, the Kjeldahl method’s use of sulfuric acid and other chemicals demands careful waste management and disposal practices to minimize environmental impact.
Advantages of Each Method
Benefits of Dumas Method
The Dumas method offers several key advantages that make it a preferred choice in many analytical settings:
- Speed: One of the most significant benefits is its quick turnaround time. Unlike other methods that require lengthy preparations and processing, the Dumas method can complete nitrogen determination in a matter of minutes per sample.
- Minimal Chemical Use: It utilizes primarily oxygen, reducing the need for hazardous chemicals, which simplifies the process and decreases the risk associated with handling and disposal.
- Automation: Modern Dumas analyzers are highly automated, reducing the potential for human error and allowing for continuous operation with minimal supervision.
- Broad Application: This method is capable of handling a wide variety of sample types, including volatile and moisture-rich samples, without the need for complex adjustments.
Strengths of Kjeldahl Method
The Kjeldahl method, while older, still stands out for several reasons:
- Accuracy: It provides exceptionally accurate results, especially in complex matrices where other elements might interfere with nitrogen measurement.
- Adaptability: It can be adjusted to accommodate different types of samples, from soil to food products to chemicals.
- Standardization: Many international standards reference the Kjeldahl method, ensuring compliance and uniformity across research and industry applications.
- Comprehensive Nitrogen Measurement: This method measures all forms of bound nitrogen, which is crucial for certain scientific and industrial applications.
Limitations and Challenges
Dumas Method Drawbacks
Despite its advantages, the Dumas method has some limitations:
- High Initial Cost: The cost of setting up a Dumas analyzer can be substantial, making it less accessible for smaller laboratories.
- Sensitivity to Sample Type: While versatile, the Dumas method can struggle with samples that have low nitrogen content or are highly heterogeneous.
- Maintenance: The combustion technology requires regular maintenance to ensure accuracy, which can add to the operational costs.
Kjeldahl Method Shortcomings
The Kjeldahl method also faces several challenges:
- Time-Consuming: The process is labor-intensive and time-consuming, requiring several hours to complete a single analysis.
- Chemical Usage: It relies on hazardous chemicals like sulfuric acid and mercury catalysts, posing risks for health and the environment.
- Waste Production: The need for neutralization and waste treatment adds to the environmental footprint and operational complexity.
Recent Advances
Technological Innovations in Dumas
Recent technological advancements have significantly improved the Dumas method:
- Enhanced Automation: New models feature advanced automation capabilities, reducing the need for operator intervention and improving reproducibility.
- Improved Sensitivity: Technological improvements have enhanced the sensitivity of nitrogen detection, allowing for more accurate analysis of trace levels.
- Eco-friendly Designs: Modern analyzers are designed to minimize energy consumption and chemical waste, aligning with sustainable laboratory practices.
Improvements in Kjeldahl
The Kjeldahl method has also seen significant innovations:
- Automated Distillation and Titration: Integration of automated systems has reduced the process time and minimized the exposure to chemicals.
- Improved Safety Measures: Modern equipment includes safety features to handle the chemicals more safely and reduce the risk of accidents.
- Enhanced Data Integration: Newer systems offer seamless integration with laboratory information management systems (LIMS), enhancing data tracking and compliance.
Choosing the Right Method
Factors to Consider
Selecting between the Dumas and Kjeldahl methods involves several considerations:
- Sample Type: The nature of the sample can dictate the choice, as certain methods may be more effective for specific sample matrices.
- Required Accuracy: If utmost precision is required, Kjeldahl might be the better option.
- Laboratory Resources: Availability of equipment, expertise, and budget can also influence the decision.
Recommendations Based on Use Case
- High-Throughput Labs: For labs needing to process large volumes of samples quickly, the Dumas method is advisable.
- Environmental and Regulatory Compliance: If compliance with certain standards is required, the Kjeldahl method may be necessary.
- Research and Development: For R&D, where accuracy over speed is preferred, the Kjeldahl method would be suitable.
FAQs
What is the Dumas Method?
The Dumas method is a nitrogen determination technique that burns a sample in pure oxygen, converting its nitrogen content into nitrogen gas. This method is valued for its speed and minimal environmental impact, as it requires fewer chemicals and produces less waste compared to other nitrogen determination methods.
How does the Kjeldahl Method work?
The Kjeldahl method begins with the digestion of the organic material using sulfuric acid, which transforms the nitrogen within the sample into ammonium sulfate. This is then alkalized and the ammonia is distilled off and quantified. This method is renowned for its accuracy and is widely used in food testing and environmental studies.
Which method is more accurate?
While both methods are highly reliable, the Kjeldahl method is often considered more accurate, especially for complex substances where other elements can interfere with the nitrogen measurement in the Dumas method. However, the choice of method can depend on specific sample requirements and laboratory capabilities.
What are the environmental impacts of these methods?
The Dumas method is generally considered more environmentally friendly due to its lower chemical usage and waste production. In contrast, the Kjeldahl method involves more hazardous chemicals and generates more waste, necessitating careful disposal and handling procedures.
Conclusion
In conclusion, the Dumas and Kjeldahl methods serve as cornerstone techniques in analytical chemistry for nitrogen determination, each with its unique set of advantages and limitations. The choice between these methods largely depends on the specific requirements of the analysis, including accuracy, efficiency, sample type, and environmental impact considerations. As analytical technology continues to advance, both methods are likely to see enhancements that improve their efficiency and reduce their environmental footprints, ensuring their ongoing relevance in scientific research and industrial applications.