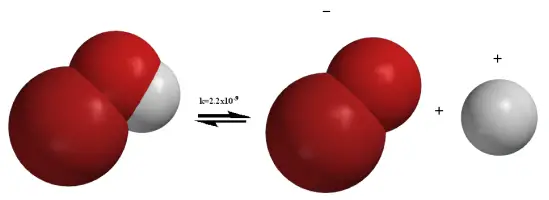
Chemical reactions are fundamental to understanding a vast array of scientific phenomena, from basic laboratory procedures to complex biological systems and industrial applications. Central to many of these reactions are two critical processes: dissociation and solvation. These mechanisms, though often discussed in tandem, serve distinct roles in the realm of chemistry.
Dissociation and solvation are key to understanding how substances interact in a solution. Dissociation refers to the process by which compounds break apart into smaller constituents, typically ions, when dissolved in a solvent. Solvation, alternatively, involves the interaction of these ions with the solvent molecules, stabilizing them in the solution and influencing their behavior and reactivity.
The distinction between these two processes is crucial for chemists and industries alike, as it affects how substances are manipulated and utilized in various contexts. For instance, the rate at which a salt dissolves in water depends significantly on both the dissociation of its ionic components and their subsequent solvation by water molecules.
Dissociation Basics
Definition of Dissociation
Dissociation in chemistry refers to the separation of molecules into simpler constituents such as atoms, ions, or radicals, typically when dissolved in a solvent. This process is fundamental to many chemical reactions, especially those involving ionic compounds like salts.
How Dissociation Occurs in Solutions
Dissociation is a critical process in the formation of solutions, particularly in the context of ionic and polar substances. Here’s a simplified step-by-step guide to how this occurs:
- Initial Contact: When a soluble ionic compound comes into contact with a solvent (like water), the solvent molecules begin to interact with the ionic compound.
- Surrounding the Ions: The polar solvent molecules surround the individual ions of the compound.
- Overcoming Ionic Bonds: The attraction between the solvent molecules and the ions becomes strong enough to overcome the ionic bonds holding the compound together.
- Formation of Ions: The compound dissociates into its constituent ions, which are then solvated (or surrounded) by solvent molecules, effectively keeping them apart and in solution.
This process is influenced by the nature of the solvent and the ionic compound, as well as environmental factors like temperature and pressure.
Solvation Explained
Definition of Solvation
Solvation, also known as dissolution, is the process of attraction and association of solvent molecules with solute ions or molecules. During solvation, solvent molecules surround solute particles and can significantly alter their properties and behavior in the solution.
Mechanism of Solvation
The mechanism of solvation involves several interactions and stages:
- Solute-Solvent Interaction: Solvent molecules approach and orient themselves around the solute particles.
- Stabilization: The interactions between solute and solvent stabilize the solute particles in the solvent, preventing them from recombining into larger particles.
- Energy Changes: Solvation involves changes in energy, including the release or absorption of heat, depending on the nature of the solvent and solute.
This mechanism is crucial for processes like the dissolution of salts in water or the absorption of gases into liquids.
Key Differences
Dissociation vs. Solvation
While both dissociation and solvation are involved in the process of forming solutions, they are distinct processes:
- Dissociation is the breakdown of a compound into smaller components, typically ions, in the presence of a solvent.
- Solvation involves the interaction of these ions with the solvent molecules, which stabilizes them in the solution.
Role in Chemical Reactions
The role of dissociation and solvation in chemical reactions is profound:
- Reactant Preparation: Dissociation provides the reactive ions necessary for the reaction.
- Reaction Medium: Solvation often determines the medium in which these reactions occur, affecting reaction rates and outcomes.
For example, in an aqueous solution, the dissociation of hydrochloric acid (HCl) into H+ and Cl- ions, followed by the solvation of these ions, is essential for the reaction with sodium hydroxide (NaOH) to form water and sodium chloride (salt).
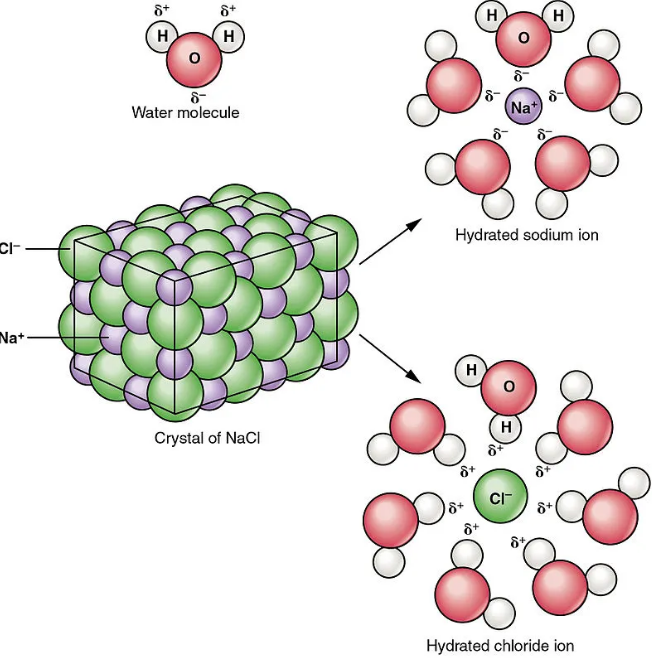
Factors Influencing Both
Impact of Solvent
The solvent plays a critical role in both dissociation and solvation processes, significantly impacting the behavior of solutes in a solution. Key points to consider include:
- Polarity of Solvent: Highly polar solvents like water are excellent at dissociating ionic compounds due to their ability to surround and stabilize ions.
- Dielectric Constant: A solvent with a high dielectric constant diminishes the electrostatic forces between ions, enhancing dissociation and improving solvation.
- Solvent Structure: The size and structure of solvent molecules can affect how well they can surround and interact with solute particles, influencing both processes.
Temperature and Pressure Effects
Temperature and pressure significantly affect dissociation and solvation, with each factor playing a role in altering the equilibrium and kinetics of these processes:
- Temperature:
- Increasing temperature generally enhances the dissociation of compounds by providing energy to overcome ionic bonds.
- Higher temperatures can also increase the solvation rate as the increased kinetic energy of solvent molecules leads to more effective solute-solvent interactions.
- Pressure:
- Higher pressures can influence solvation by affecting the solvent’s density and the solubility of solutes.
- Pressure changes can also shift the equilibrium of dissociation, especially in systems where gases are involved.
Real-World Applications
Industrial Uses
Dissociation and solvation processes have a wide range of applications in various industries:
- Pharmaceuticals: Control of these processes is crucial in drug formulation where the solubility and bioavailability of medications depend on effective solvation and dissociation.
- Chemical Manufacturing: Many synthesis reactions require precise control of solute interactions, which are governed by dissociation and solvation dynamics.
- Water Treatment: Dissociation is essential for the removal of contaminants through processes like ion exchange, while solvation dynamics are crucial in solvent extraction methods.
Biological Implications
In biological systems, dissociation and solvation are fundamental to numerous processes:
- Cellular Function: Many cellular processes, such as enzyme activity and signal transduction, depend on the dissociation of molecules and the solvation of ions to function correctly.
- Nutrient Absorption: The solubility and transport of nutrients, vitamins, and minerals in the human body are heavily influenced by these chemical processes.
- Neurotransmission: Neurotransmitter activity involves the rapid dissociation and solvation of molecules across synapses, crucial for nerve signal transmission.
FAQ
What is dissociation in chemistry?
Dissociation in chemistry refers to the process where molecules or ionic compounds separate or split into smaller particles, usually ions, when dissolved in a solvent. This process is fundamental in numerous chemical reactions and is essential for the conductivity of electrolytic solutions.
How does solvation differ from dissolution?
Solvation involves the interaction of solvent molecules with dissolved particles, such as ions or molecules, stabilizing them in a solution. It is different from dissolution, which is the overall process of a solute dissolving in a solvent, which may include both dissociation and solvation.
Why is understanding solvation important?
Understanding solvation is crucial because it affects the solubility, reactivity, and transport properties of substances in a solution. It plays a vital role in fields ranging from pharmacology, where it influences drug delivery, to environmental science, affecting the mobility of pollutants.
Can dissociation occur without solvation?
While theoretically possible, in practical scenarios, dissociation without solvation is rare, as solvation typically follows dissociation immediately, especially in polar solvents like water. This immediate interaction often stabilizes the dissociated ions.
Conclusion
In conclusion, dissociation and solvation are integral to the study and application of chemistry. They not only explain how substances behave in solutions but also influence various industrial and biological processes. Recognizing the differences between these two processes is essential for manipulating chemical reactions and achieving desired outcomes in both laboratory and industrial settings.
These insights into dissociation and solvation not only provide a foundation for academic and professional pursuits in chemistry but also enhance the practical understanding necessary for innovative applications in technology and medicine.