Decantation and filtration are two fundamental processes used across various industries for separating mixtures and purifying substances. These techniques leverage physical differences between components, such as density and particle size, to achieve separation. While both methods are aimed at clarifying and purifying substances, they operate on distinct principles and are suitable for different types of mixtures.
Decantation is a process that separates mixtures by removing a layer of liquid from another that has settled sediments, relying on gravity to differentiate between substances based on density. Filtration, on the other hand, involves passing a mixture through a filter, which retains the solid particles while allowing the liquid to pass through. This method is effective for mixtures where solid particles are suspended in a fluid.
The choice between decantation and filtration depends on the specific requirements of the purification process, including the nature of the mixture, desired purity level, and efficiency considerations. Decantation is often used when dealing with immiscible liquids or when solid particles have settled at the bottom of a container, while filtration is preferred for mixtures of fine particles dispersed in a liquid or gas. Understanding these differences is crucial for selecting the most appropriate method for a given application, ensuring effective separation and purification.
Basic Concepts
What is Decantation?
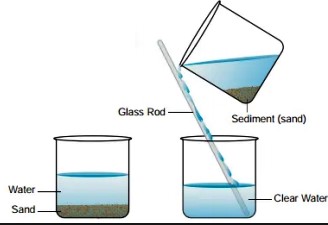
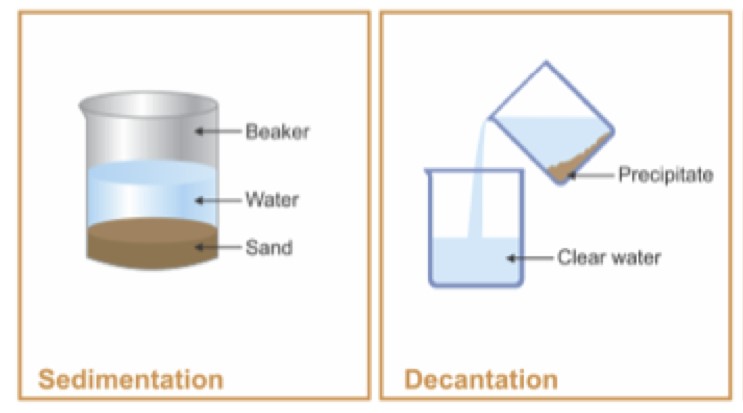
Decantation is a separation technique used to separate mixtures of liquids and solids or two immiscible liquids. The principle behind decantation relies on gravity to pull the denser substance to the bottom, allowing the less dense liquid to be poured off from the top. This method is often used when a quick and straightforward separation is required, where the difference in density between substances is significant.
Common applications of decantation include:
- Separating sand and water: A classic example used in schools to demonstrate the concept.
- Clarifying liquids in cooking, such as removing fat from broth.
- In laboratories, to separate precipitates from reaction mixtures.
- Oil and water separation in the environmental management industry.
What is Filtration?
Filtration is a process used to separate solids from liquids or gases by passing the mixture through a medium (filter) that retains the solid particles while allowing the fluid to pass through. The principle of filtration is based on particle size, with the filter acting as a barrier to substances larger than its pores.
Common applications of filtration include:
- Water purification systems to remove impurities and contaminants.
- Air filtration in HVAC systems to improve indoor air quality.
- In the pharmaceutical industry, to sterilize liquids by removing bacterial contaminants.
- Coffee making, where the coffee grounds are separated from the liquid brew.
Key Differences
Process Mechanism
How decantation works
Decantation involves:
- Allowing the mixture to sit undisturbed.
- The denser particles or liquid settles at the bottom.
- The upper layer of liquid is carefully poured off into another container.
How filtration works
Filtration involves:
- Passing the mixture through a filter.
- Solid particles are trapped by the filter.
- The liquid or gas passes through the filter.
Equipment Used
Tools for decantation
- Separatory funnels: Used for liquid-liquid separations.
- Decanting basins: Often used in laboratories for simple solid-liquid separations.
Tools for filtration
- Filter papers and membranes: Come in various pore sizes for different applications.
- Vacuum filtration apparatus: Uses reduced pressure to speed up the filtration process.
Application Areas
Industries relying on decantation
- Environmental management: For oil spill cleanup and wastewater treatment.
- Food and beverage: To clarify wines and broths.
Industries relying on filtration
- Pharmaceuticals: For sterile drug preparations.
- Water treatment: In both municipal and industrial settings for purifying water.
Efficiency and Precision
Comparison in terms of efficiency
- Decantation is less efficient with mixtures where the particles are very small or do not settle easily.
- Filtration can be highly efficient, especially with the right choice of filter material and pore size, even for fine particles.
Comparison in terms of precision
- Decantation might result in some cross-contamination between the layers due to the manual pouring process.
- Filtration tends to be more precise, as the filter can effectively separate particles based on size.
Time and Cost
Time considerations for both methods
- Decantation usually requires waiting for the substances to naturally separate, which can be time-consuming.
- Filtration can be quick, especially with the aid of pressure or vacuum systems, but depends on the filter’s pore size and the mixture’s viscosity.
Cost implications for both methods
- Decantation generally requires minimal equipment, making it a cost-effective option for simple separations.
- Filtration can involve more specialized and therefore expensive equipment, especially for high-precision or industrial-scale processes.

When to Use Which?
Decantation Scenarios
Ideal conditions for decantation
Decantation is most effective when:
- The density difference between the liquid and solid (or two liquids) is significant, allowing for quick settling.
- The mixture contains large particles or a clear liquid layer that can easily separate by gravity.
- You’re dealing with a large volume of mixture where filtration might be impractical or too time-consuming.
Limitations of decantation
However, decantation has its drawbacks:
- It’s not effective for fine particles that remain suspended in the liquid.
- Cross-contamination is possible since the separation is not always clear-cut.
- It requires patience, as waiting for particles to settle can be time-consuming, especially in large volumes.
Filtration Scenarios
Ideal conditions for filtration
Filtration shines when:
- Dealing with fine particles that cannot easily settle by gravity.
- Clarity and purity of the liquid are paramount, and any contamination is unacceptable.
- The process requires speed, especially when using pressure-driven filtration methods.
Limitations of filtration
Filtration also has its limitations:
- It can be costly due to the need for specific filters and sometimes expensive equipment.
- Clogging can occur, especially with very fine particles or high volumes, necessitating frequent maintenance or replacement of filters.
Advancements and Innovations
Technological Advancements in Decantation
Recent developments
In recent years, decantation has seen improvements through the use of automated decanting systems. These systems use sensors to detect the separation layers and precisely control the decanting process, reducing the risk of cross-contamination and improving efficiency.
Future prospects
Looking ahead, smart decantation systems with enhanced sensing technologies are expected to become more prevalent. These systems could offer real-time adjustments based on the characteristics of the mixture, further optimizing the separation process.
Technological Advancements in Filtration
Recent developments
Filtration technology has significantly advanced with the introduction of nano-filtration and ultra-filtration systems. These methods use membranes with extremely small pore sizes, capable of removing microscopic contaminants, including viruses and certain chemicals.
Future prospects
The future of filtration looks promising with the exploration of graphene-based filters. Graphene, with its exceptional strength and thinness, could revolutionize filtration by providing ultra-efficient barriers against even the smallest of particles, while maintaining high flow rates.
Environmental Impact
Sustainability Factors
Environmental benefits of decantation
Decantation, being a passive separation technique, offers benefits such as:
- Low energy consumption, as it primarily relies on gravity.
- Minimal use of additives or chemicals, making it a greener option for separating mixtures.
Environmental benefits of filtration
Filtration contributes to environmental protection by:
- Providing a means to purify water and air, removing harmful pollutants and contaminants.
- Enabling the recycling and reuse of materials, as purified substances can often be returned to the environment safely or used again.
Waste Management
Handling waste in decantation
Waste management in decantation involves:
- Settling tanks for large-scale operations, where solids collect at the bottom and are periodically removed.
- Disposal of the separated solid waste according to environmental regulations, ensuring minimal environmental impact.
Handling waste in filtration
In filtration, waste management includes:
- Regular replacement or cleaning of filters to maintain efficiency and prevent clogging.
- Safe disposal or recycling of used filters, especially those containing hazardous materials, to prevent environmental harm.
Frequently Asked Questions
What is the main difference between decantation and filtration?
The main difference lies in their separation mechanisms. Decantation separates substances based on density, allowing heavier sediments to settle before the top layer is removed. Filtration, however, separates based on particle size, using a filter to retain solid particles while letting the fluid phase pass through.
When should I use decantation instead of filtration?
Decantation is ideal for separating mixtures of liquids with different densities or when a solid has settled at the bottom of a liquid and can be easily separated by gently pouring the top liquid away. It’s less suitable for mixtures with fine particles that do not settle quickly.
Can decantation and filtration be used together?
Yes, decantation and filtration can be used in tandem for more complex purification tasks. Decantation may first be employed to separate larger, settled particles or to partially separate immiscible liquids, followed by filtration to remove finer particles, achieving a higher purity level.
How does particle size affect filtration efficiency?
The efficiency of filtration largely depends on the size of the particles and the pore size of the filter. A filter with pores small enough to capture particles provides more effective separation. However, if the particles are too small or the filter’s pore size is too large, some particles may pass through, reducing efficiency.
Conclusion
The decision to use decantation or filtration in a separation process is influenced by the specific characteristics of the mixture and the desired outcome of the purification process. Decantation is best suited for quick separations of mixtures with clear density differences or when solid particles have settled, while filtration excels in removing fine particles from fluids.
Understanding the principles behind decantation and filtration allows for the selection of the most effective method for each unique situation. By leveraging these techniques appropriately, industries can achieve optimal purity levels, ensuring the quality and safety of their products. As technology advances, the efficiency and applicability of these methods continue to improve, highlighting their enduring importance in separation science.